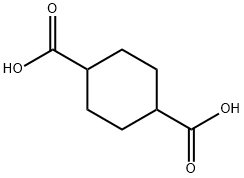
Research Progress on 1,4-Cyclohexanedicarboxylic Acid: Structure, Polymers, and Functional Applications
Introduction
The chemical structure of 1,4-cyclohexanedicarboxylic acid offers the resin and polymer industry a unique diacid intermediate. The heart of the molecule is a 1,4-substituted cyclohexane ring. The cycloaliphatic structure is responsible for many of the desirable performance properties that 1,4-cyclohexanedicarboxylic acid imparts to polyester resins and enamels. Compared with aromatic and aliphatic diacids, some of the benefits of using 1,4-cyclohexanedicarboxylic acid are that it offers an incremental improvement in hardness/flexibility ratio over a traditional 1:1 blend of hard aromatic and flexibilizing aliphatic diacids. The stain/detergent/salt spray resistance benefits are especially useful in appliance-grade coil coatings. Resins containing 1,4-cyclohexanedicarboxylic acid are also used in stabilized exterior coil coatings. [1]

1,4-Cyclohexanedicarboxylic Acid' s cis-trans Structure
1,4-cyclohexanedicarboxylic acid is a commonly used monomer in the synthesis of polyesters and polyamides. Information on the influence of the synthesis conditions and/or thermal treatment of the polymers on the cis–trans ratio of the incorporated 1,4-cyclohexanedicarboxylic acid is scarce. This cis–trans ratio largely influences the physical properties of the polymers. Compared to the cis isomer, incorporation of the trans isomer apparently leads to more stable crystals. It can be expected that the cis isomer introduces ‘kinks’ into the main chain, which may hinder crystallization. [2]
Melting Behavior of Nylon 6.6 with 1,4-Cyclohexanedicarboxylic Acid
In a study of the effects of incorporating ring structures (aromatic and/or aliphatic) on the structure–property relations of nylon 6.6 copolyamides, it appears that partial substitution of adipic acid by 1,4-cyclohexanedicarboxylic acid results in a rise in melting temperature of the copolyamides. One would expect that this partial substitution would lead to a decrease of Tm because of the disturbance of chain regularity. This rise in Tm is even more pronounced than the one observed for an identical degree of substitution of the adipic acid by terephthalic acid (TA), indicating a better accommodation of the 1,4-cyclohexanedicarboxylic acid residue in the crystal lattice as compared to a TA residue. These copolyamides were synthesized using a cis–trans mixture of 1,4-cyclohexanedicarboxylic acid, and it was assumed that all 1,4-cyclohexanedicarboxylic acid units became trans during synthesis. [2]
1,4-Cyclohexanedicarboxylic Acid–based Polymers
Polyanhydrides for drug-controlledrelease systems from 1,4-cyclohexanedicarboxylic acid were synthesized by melt polycondensation. The degradation of polymers was estimated by weight loss in 0.1 mol l-1, pH 7.4 phosphate buffer at 37℃. The drug delivery was conducted in the same buffer. The results show that the different polymers lost weight over 150– 360 h, and fine surface erosion was investigated. The different conformation of 1,4-cyclohexanedicarboxylic acid has an obvious influencce on the degradation of polyanhydrides due to their different crystallinity, with higher crystallinity samples degrading much more slowly. The incorporation of adipic acids into the poly 1,4-cyclohexanedicarboxylic acid can obviously accelerate the degradation and the introduction of -CH2- segments increased the flexibility of polyanhydride backbone and accelerated the degradation rate. In vitro delivery experiments show that Bruffen was completely released in about 10 days from a melting mold disk with a fine linear delivery curve. [3]
Metal–organic Frameworks
Metal-organic coordination polymers (MOCP) belong to a relatively new but very important class of hybrid materials and are of significant interest for both fundamental and applied research in connection with a wide range of their functional properties. Functionalized derivatives of cyclic or polycyclic (caged) saturated hydrocarbons, such as 1,4-cyclohexanedicarboxylic acid, can be the optimal organic ligands. These molecules can act as bridging ligands, while having sufficient conformational stability to ensure the stability of the porous framework with incorporated photoactive molecules. In the present work, structural and physico-chemical characterization of three new coordination polymers based on trans-1,4-cyclohexanedicarboxylic acid were synthesized , namely, (H2chdc)):[Mn3(chdc)3-(NMP)2(DMF)2] (1), [Zn3(chdc)3(NMP)2]•2NMP (2), and [Zn 3(chdc)3(ur)(DMF)0.5]•DMF (3), which are promising in terms of their possible application as UV transparent matrices for carrying out photochemical transformations in their pores. [4]
References:
[1] https://www.eastman.com/Literature_Center/N/N327.pdf?
[2] Vanhaecht, B., Teerenstra, M. N., Suwier, D. R., Willem, R., Biesemans, M., & Koning, C. E. (2001). Controlled stereochemistry of polyamides derived from cis/trans‐1, 4‐cyclohexanedicarboxylic acid. Journal of Polymer Science Part A: Polymer Chemistry, 39(6), 833-840.
[3] Zhang, T., Gu, M., & Yu, X. (2001). Degradation and drug delivery properties of poly (1, 4-cyclohexanedicarboxylic anhydride). Journal of Biomaterials Science, Polymer Edition, 12(5), 491-501.
[4] Demakov, P. A., Sapchenko, S. A., Samsonenko, D. G., Dybtsev, D. N., & Fedin, V. P. (2018). Coordination polymers based on zinc (II) and manganese (II) with 1, 4-cyclohexanedicarboxylic acid. Russian Chemical Bulletin, 67(3), 490-496.
You may like
See also
Lastest Price from 1,4-Cyclohexanedicarboxylic acid manufacturers

US $0.00/KG2025-04-21
- CAS:
- 1076-97-7
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1.00/KG2025-04-21
- CAS:
- 1076-97-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt