Synthesis and Bioactivity of Lapatinib
General description
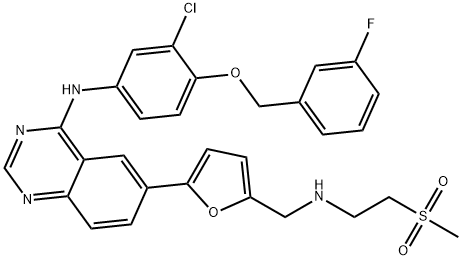
Lapatinib, which is a chemical. Chemical name N-[3-chloro-4 -[(3-fluorophenyl) methoxy] phenyl]-6-[5-[(2-methylsulfonyl ethyl amino) methyl] -2-furanyl] quinazoline-4-amine, molecular formula is C29H26ClFN4O4S, molecular weight is 581.05800. Lapatinib is an antitumor agent that has been clinically used in combination with capecitabine for the treatment of erbb-2 overexpression in patients with advanced or metastatic breast cancer who have previously received treatment including anthracycline, paclitaxel and trastuzumab (herceptin).
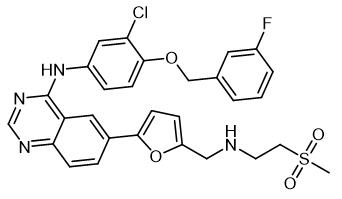
Fig. 1 The structure of Lapatinib.
Physicochemical property
Lapatinib is a yellow solid with a melting point of 144-146℃. It is soluble in DMSO (up to 200 mg/mL).
Synthetic routes
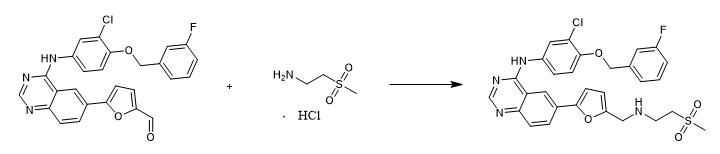
Fig. 2 The synthetic scheme 1 of Lapatinib.
To a reaction flask were added 2-(methylsulfonyl)ethylamine?HCl (0.75 mmol), methanol (5.0 mL), anhydrous sodium sulfate (2.00 mmol) and triethylamine (0.5 mL) cooled to 0 °C. The mixture was stirred 20 min and adjusts pH~5-6 with formic acid. The reactor was added the solution of 4-arylamino-6-(5-formylfuran-2-yl)quinazoline (D1) (0.50 mmol) resolved in THF (5 mL) and DMF (5 mL). Sodium cyanoborohydride (2.00 mmol) was added in two portions over a 20 min period. After stirring for 2 h, the precipitated product was filtered and the filtrate was evaporated. The crude product was chromatographed by silica gel, eluted with MeOH/CHCl3 (1:15) to afford as pale yellow solid (yield 82.0%).1H NMR (600 MHz, DMSO-d6) δ(ppm): 9.91 (s, 1H), 8.74 (s, 1H), 8.57 (s, 1H), 8.16 (dd, J = 8.7, 1.5 Hz, 1H), 8.04 (d, J = 2.5 Hz, 1H), 7.81 (d, J = 8.7 Hz, 1H), 7.77 (dd, J = 9.0, 2.5 Hz, 1H), 7.48 (dd, J = 14.0, 7.8 Hz, 1H), 7.37 – 7.31 (m, 2H), 7.29 (d, J = 9.0 Hz, 1H), 7.23 – 7.17 (m, 1H), 7.06 (d, J = 3.2 Hz, 1H), 6.51 (d, J = 3.2 Hz, 1H), 5.27 (s, 2H), 3.86 (s, 2H), 3.31 (t, J = 6.7 Hz, 2H), 3.06 (s, 3H), 3.03 (t, J = 6.7 Hz, 2H), 1.93 (s, 1H) [1].
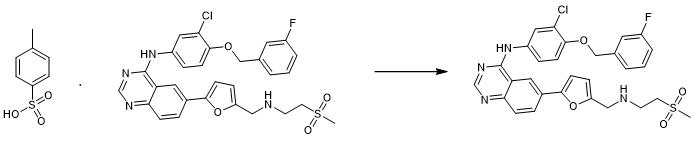
Fig. 3 The synthetic scheme 2 of Lapatinib.
To the yellow suspension of 125 mg of lapatinib ditosylate (the active component of the drug Tykerb, CAS number 388082-78-8) in 10 mL of ethyl acetate 10 mL of aqueous solution of sodium carbonate (0.1 mg/mL) was added. The mixture was stirred at room temperature for 60 min during which time it changed into colorless. Then the upper organic layer was separated and washed twice with 10 mL of water at 40–50 °C. The volume of organic phase containing lapatinib in a form of free base was reduced in rotary evaporator and the product was lyophilized, yield ≈ 85%. EA: calc. %: C 59.95; H 4.51; N 9.64; S 5.52; found %: C 60.15; H 4.38; N 9.81; S 5.63; MS: m/z: calc. 581.07; found 581.09 [M + H+]. 1H NMR: (CD3CN) δ (ppm): 3.04 (s, 3H, CH3–S); 3.55 (m, 2H, CH2–CH2–N); 3.65 (m, 2H, S–CH2–CH2); 5.26 (s, 2H, O–CH2–C); 6.90 and 7.21 (respectively, d, 1H, C–CH–CH–C and d, 1H, C–CH–CH–C, in aromatic ring of furan), 7.15, 7.29, 7.33 and 7.47 (respectively, ddd, 1H, C(F)–CH–CH–CH C, d, 1H, C–CH–C(F), m, 1H, C(F)–CH–CH–CH–C and m, 1H, C(F)–CH–CH–CH–C, in aromatic ring of fluorobenzene), 7.20, 7.44 and 7.69 (respectively, d, 1H, C(Cl)–C–CH–CH, dd, 1H, C–CH–CH–C and d, 1H, C(N)–CH–C(Cl) in aromatic ring of chlorobenzene), 8.93, 8.23 and 8.15 (respectively, s, 1H, C–CH–C, dd, 1H, C–CH–CH and d, 1H, CH–CH–C, in aromatic ring of benzene), 8.68 (s, 1H, N–CH–N); 8.91 (s, 2H, NH–CH2–C); 10.99 (s, 1H, C–NH–C) [2].
Antitumor activity
Breast Cancer
Overexpression of the ERBB2 kinase is observed in about one-third of breast cancer patients and the dual ERBB1/ERBB2 kinase inhibitor lapatinib was recently approved for the treatment of advanced ERBB2-positive breast cancer. Mutations in the ERBB2 receptor have recently been reported in breast cancer at diagnosis and also in gastric, colorectal and lung cancer. These mutations may have an impact on the clinical responses achieved with lapatinib in breast cancer and may also have a potential impact on the use of lapatinib in other solid cancers. However, the sensitivity of lapatinib towards clinically observed ERBB2 mutations is not known. Duyster et al. cloned a panel of 8 clinically observed ERBB2 mutations, established stable cell lines and characterized their sensitivity towards lapatinib and alternative ERBB2 inhibitors. Both lapatinib-sensitive and lapatinib-resistant ERBB2 mutations were observed. Interestingly, they were able to generate lapatinib resistance mutations in wt-ERBB2 cells incubated with lapatinib for prolonged periods of time. This indicates that these resistance mutations may also cause secondary resistance in lapatinib-treated patients. Lapatinib-resistant ERBB2 mutations were found to be highly resistant towards AEE788 treatment but remained sensitive towards the dual irreversible inhibitors CL-387785 and WZ-4002. Patients harbouring certain ERBB2 kinase domain mutations at diagnosis may not benefit from lapatinib treatment. Moreover, secondary lapatinib resistance may develop due to kinase domain mutations. Irreversible ERBB2 inhibitors may offer alternative treatment options for breast cancer and other solid tumor patients harbouring lapatinib resistance mutations. In addition, these inhibitors may be of interest in the scenario of secondary lapatinib resistance [3].
The aqueous solubility of lapatinib declines significantly at pH>4, suggesting that its bioavailability might be lowered by acid-reducing drugs. A study was therefore conducted to assess the effects of esomeprazole on lapatinib pharmacokinetics (PK). Women with metastatic human epidermal growth factor receptor 2 positive (HER2+) breast cancer were enrolled. Patients received 1,250 mg lapatinib once daily (QD) in the morning on Days 1-7 (Period 1) and Days 8-14 (Period 2) with 40 mg esomeprazole QD at bedtime 3 hours after dinner on Days 8-14. Lapatinib PK sampling occurred during the 24-hour steady-state dosing intervals on Day 7 (lapatinib alone) and Day 14 (lapatinib with esomeprazole). Esomeprazole treatment resulted in decreased lapatinib bioavailability (mean 26%, range 6-49%) that was inversely associated with patient age as a significant covariate [4].
Gastric Cancer
Lapatinib is a small-molecule tyrosine kinase inhibitor that plays important roles in cell proliferation and survival. Administration of lapatinib with capecitabine is an effective treatment for HER2-positive metastatic BC. However, the effects of lapatinib on gastric cancer (GC) remain to be clear. In this study, we aimed to investigate the therapeutic effects of lapatinib combined with sulforaphane on GC and its underlying mechanisms. Methods. SGC-7901 and lapatinib-resistant SGC-7901 cells were treated with lapatinib (0.2 mu M), sulforaphane (5 mu M), or their combinations. Cell viability, invasion, cycle, and apoptosis of SGC-7901 and lapatinib-resistant SGC-7901 cells were evaluated by thiazolyl blue tetrazolium bromide (MTT), Boyden chamber assay, and flow cytometer. The protein expressions of HER-2, p-HER-2, AKT, p-AKT, ERK, and p-ERK were detected by Western blotting
You may like
Related articles And Qustion
See also
Lastest Price from Lapatinib manufacturers

US $0.00-0.00/mg2025-06-28
- CAS:
- 231277-92-2
- Min. Order:
- 10mg
- Purity:
- 99%+ HPLC
- Supply Ability:
- 1000

US $5.00-0.50/KG2025-06-05
- CAS:
- 231277-92-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS