Synthesis and Application of 4-methoxybenzylamine
General description
The molecular formula of 4-methoxybenzylamine is C8H11NO, the molecular weight is 137.18, and the cas number is 2393-23-9. 4-methoxybenzylamine is an organic amine compound and an important pharmaceutical intermediate.
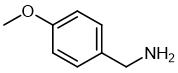
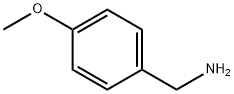
Fig. 1 The structure of 4-Methoxybenzylamine.
Physicochemical property
4-methoxybenzylamine is a clear colorless to slightly yellow liquid with a melting point of -10°C and a boiling point of 236-237 °C(lit.). It is stable at normal temperature and pressure. It needs to be stored in an airtight container in a cool, dry, well-ventilated place away from incompatible substances. It dissolves easily in water.
Synthetic routes
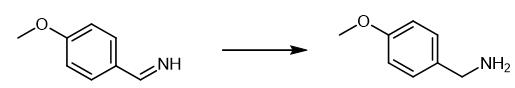
Fig. 2 The synthetic scheme 1 of 4-Methoxybenzylamine.
Take substituted phenylmethanamine (0.05 mmol) and palladium nanoparticle (2.0 mg, 13 mol%) in an oven dried reaction bottle (25 ml) equipped with magnetic pellet. Add H2O (10 ml, pH = 2.0) to the reaction tube. Stir the reaction mixture at room temperature in 1 hour with H2 balloon. Monitor the reaction mixture by TLC. Stop the reaction mixture. Extract the solution with saturated salt water and ethyl acetate (3×10 ml). Dry the organic phase by using Na2SO4.Concentrate the organic phase in vacuum. Purify the product by column chromatography using silica gel. 1H NMR (400 MHz, DMSO-d6): δ = 7.24 (d, J= 8.0 Hz, 2H), 6.86 (d, J= 8.0 Hz, 2H), 3.72 (s, 3H), 3.65 (s, 2H), 1.68 (brs, 2H). 13C NMR (100 MHz, DMSO-d6): δ = 158.25, 136.82, 128.56, 113.91, 55.41, 45.60 [1].

Fig. 3 The synthetic scheme 2 of 4-Methoxybenzylamine.
Take substituted benzaldehyde (0.05 mmol), ammonia water (0.2 mmol), amine and Pd (2.0 mg) in an oven dried reaction bottle (25 ml) equipped with magnetic pellet. Add H2O (10 ml, different pH, 2.0) to the reaction tube. Stir the reaction mixture at room temperature for 3 hours with H2 balloon. Monitor the reaction mixture by TLC. 1H NMR (400 MHz, DMSO-d6): δ = 7.24 (d, J= 8.0 Hz, 2H), 6.86 (d, J= 8.0 Hz, 2H), 3.72 (s, 3H), 3.65 (s, 2H), 1.68 (brs, 2H). 13C NMR (100 MHz, DMSO-d6): δ = 158.25, 136.82, 128.56, 113.91, 55.41, 45.60 [1].
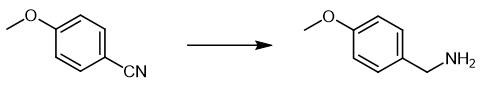
Fig. 4 The synthetic scheme 3 of 4-Methoxybenzylamine.
Dissolve 4-methoxybenzonitrile (0.5 mmol), H3N·BH3 (32 mg, 1 mmol), tetraethylsilane (10 μL, 0.05 mmol) and 1(η2-NCMe) (5 mg, 0.01 mmol) in 1 mL THF in a 10 mL Schlenk tube. Stir the reaction mixture at room temperature for 8 hours. Dissolve a sample of the reaction mixture (100 μL) in 0.5 mL of CDCl3. Filter the reaction mixture through Celite. Add 0.5 mL (or 1.0 mL for the hydrogenation of 3f) of a HCl solution (1.0 M in Et2O) to the collected filtrate, which resulted in a white precipitate immediately. Wash the product with DCM. Extract the product with MeOH. Dry the product [2].
Application
1. 4-methoxy benzylamine can be used as raw material for the preparation of 3-chloro-4-methoxy benzylamine hydrochloride. 3-chloro-4-methoxybenzylamine hydrochloride is an intermediate side chain for the synthesis of avanafil. Avanafil is an oral, fast-acting, highly selective phosphodiesterase-5 (PDE-5) inhibitor for the treatment of erectile dysfunction in men. Its original research was developed by Tanabe Mitsubishi Pharmaceutical Co., LTD., and its development was authorized by Vivus In the United States. In April 2012, Avanafil was approved for marketing in the United States. Compared with competing products, avanafil has obvious advantages in its faster onset.
2. In a study of NaH-promoted aza-Michael reaction of equimolar amounts of unsaturated malonate 1 with 4-methoxybenzylamine 2 with incomplete consumption of the reactants, authors observed that two unexpected products were formed, namely, tetraester 3 and imine 4 (Fig. 4). Obviously, compounds 3 and 4 are the products of intermolecular disproportionation of the starting compounds 1 and 2 [3].
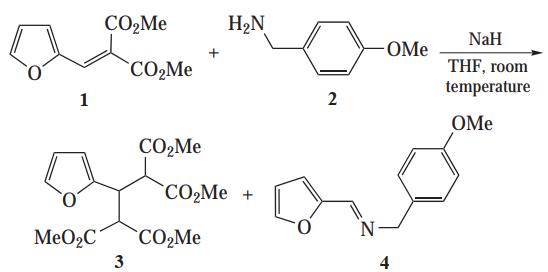
Fig. 4 Intermolecular disproportionation.
References
[1] Jv X, Sun S, Zhang Q, et al. Efficient and mild reductive amination of carbonyl compounds catalyzed by dual-function palladium nanoparticles[J]. ACS Sustainable Chemistry & Engineering, 2019, 8(3): 1618-1626.
[2] Hou S F, Chen J Y, Xue M, et al. Cooperative molybdenum-thiolate reactivity for transfer hydrogenation of nitriles[J]. ACS Catalysis, 2019, 10(1): 380-390.
[3] Torosyan S A, Biglova Y N, Gimalova F A, et al. Intermolecular disproportionation between dimethyl (2-furylmethylidene) malonate and 4-methoxybenzylamine[J]. Mendeleev Communications, 2016, 26(5): 429-430.
Related articles And Qustion
See also
Lastest Price from 4-Methoxybenzylamine manufacturers

US $0.00-0.00/kg2025-06-30
- CAS:
- 2393-23-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 2393-23-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt