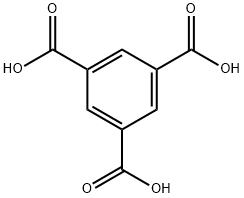
Synthesis and Application of Trimesic acid
General description
Trimesic acid, or 1,3,5-Benzenetricarboxylic acid, is a carboxylic acid derivative, which can be used as intermediate in organic synthesis. It is an important chemical raw material, which is of great significance to the production of plastics, artificial fibers, water-soluble alkyl resins and plasticizers. It is also used for the preparation of fungicide,mildew inhibitor, plasticizer and cross-linking agent.
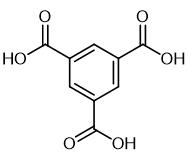
Fig. 1 The structure of Trimesic acid.
Physicochemical property
Tryptamine is a white to yellowish crystalline powder with a melting point of 380℃. It has a flash point of 328℃. It is soluble in ethanol and ether, and soluble in 40 parts of water at 23℃.
Synthetic routes
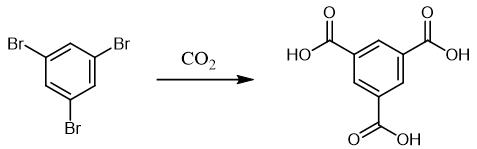
Fig. 2 The synthetic scheme 1 of Trimesic acid.
To a mixture of Cu(OAc)2.H2O (0.02 mmol, 2 mol%) and 1,2- bis(diphenylphosphinyl)benzene (0.03 mmol, 3 mol%) in an oven dried Schlenk tube connected to a CO2 balloon, a solution of PMHS (polymethylhydrosiloxane, 4.0 mmol, 4.0 equiva.) in 1,4-dioxane (3.0 mL) was added, flushed the tube with CO2, heated the resulting blue solution to 65 ℃ while vigorously stirring for 25 minutes under CO2 balloon pressure and the solution becomes light brown. This formate solution was removed from the heating bath and added toluene (12 ml), aryl dihalide (1.0 mmol, 1.0 equiv.), Pd(OAc)2 (5 mol%, 0.05 mmol), xantphos (10 mol%, 0.1 mmol) and Et3N (4.0 mmol, 4.0 equiva.) sequentially under CO2 atmosphere. The Schlenk tube was closed, CO2 balloon was removed, and heated to 100 ℃ for the time 20 h. Then, the reaction mixture was cooled to ambient temperature and added 20 ml of 1N NaOH and stirred for 10 min. The resulting emulsion was filtered and the filtrate was extracted with EtOAc (3*20 mL), aqueous portion separated was acidified with 1.0 N HCl or 1M citric acid, solid precipitated was filtered, suck dried to get the pure diacid. If no precipitation occurs, extracted with EtOAc (3*30 mL) and these organic fractions were dried over Na2SO4 and concentrated to get the pure compound. [1].
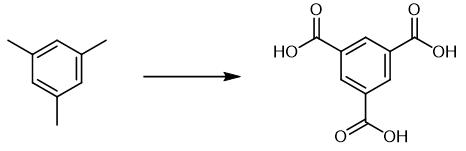
Fig. 3 The synthetic scheme 2 of Trimesic acid.
A 25mL quartaz glass tube was charged with a stiring bar, and added 1g (0.1 mmol, 12.0 mg), CeCl3 (5 mol%, 1.2 mg), CCl3CH2OH 1.0 equiv, 14.9 mg), CH3CN (2 mL) under O2 atmosphere. The tube was perpendicular to a 400 nm LED light (10 W), and the mixture was stirred under the irradiation of blue light for 72 hours at 60 ℃. After completion of reaction as indicated by TLC. Subsequently, the solvent was concentrated under reduced pressure, and the residue was purified directly by washing with acetonitrile to give the corresponding product. 17.7 mg, 84%. 1H NMR (400 MHz, DMSO-d6) δ 13.54 (brs, 3H), 8.63 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 166.3, 134.0, 132.4. [2].
Application
1. The covalent symmetrization of the hexagonal networks of 1,3,5-trimesic acids under compression was studied applying inelastic Raman spectroscopic scattering in the pressure range 0–7.6 GPa. The first pressure induced phase transition, resulted from representative warping of trimesic acids network plane, was observed when compression met 0.4 GPa. Above approximately 1.9 GPa, as reflected in our Raman data, intra-plane hydrogen bonds became covalent and proton became homogeneous. Further compression beyond 7.6 GPa led to a significant loss of long-range order, marked by amorphization. Pressure-induced transformation and its mechanisms, exhibited in our experiment, reflect a characteristic structural transition of symmetric trimesic acids resulted from the effect of hydrogen-bonding interaction. [3].
2. Mahiuddin et al. investigated the adsorption kinetics, adsorption isotherms and surface complexation of trimeric acid on α -alumina. Adsorption kinetics of trimesic acid with an initial concentration of 0.5 mM onto α-alumina surfaces were carried out in batch method in presence of 0.05 mM NaCl(aq) at pH 6 and 298.15, 303.15 and 313.15 K. Adsorption isotherms were carried out at 298.15 K, pH 5–9, and 0.05 mM NaCl(aq) by varying trimesic acid concentration from 0.01 to 0.6 mM. Three kinetics equations such as pseudo-first-order, pseudo-second-order and Ho equations were used to estimate the kinetics parameters of the adsorption of trimesic acid on the α-alumina surfaces. Ho equation fits the experimental kinetics data significantly better and the estimated equilibrium concentration is in excellent agreement with the experimental value. The adsorption data were fitted to Freundlich and Langmuir adsorption model and the later best fits the adsorption isotherms. Comparison of adsorption density of trimesic acid with that of benzoic and phthalic acids follows the sequence: benzoic acid < trimesic acid < phthalic acid. The negative activation energy and the Gibbs free energy for adsorption indicate that the adsorption of trimesic acid onto α-alumina is spontaneous and facile. DRIFT spectroscopic studies reveal that trimesate forms outer-sphere complexes with the surface hydroxyl groups that are generated onto α-alumina surfaces in the pH range of the study [4].
References
[1] Paridala K, Lu S M, Wang M M, et al. Tandem one-pot CO 2 reduction by PMHS and silyloxycarbonylation of aryl/vinyl halides to access carboxylic acids[J]. Chemical Communications, 2018, 54(82): 11574-11577.
[2] Wang C C, Zhang G X, Zuo Z W, et al. Photo-induced deep aerobic oxidation of alkyl aromatics[J]. Science China Chemistry, 2021, 64(9): 1487-1492.
[3] Li J, Zhao Z, Ren P, et al. Covalent symmetrization of the hexagonal networks of trimesic acids at high pressure[J]. Optik, 2016, 127(13): 5396-5399.
[4] Borah J M, Mahiuddin S. Adsorption and surface complexation of trimesic acid at the α-alumina–electrolyte interface[J]. Journal of colloid and interface science, 2008, 322(1): 6-12.
See also
Lastest Price from Trimesic acid manufacturers
US $0.00-0.00/kg2025-12-02
- CAS:
- 554-95-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00/KG2025-04-21
- CAS:
- 554-95-0
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month