Is acetylene a polar molecule?
Overview
Ethyne, also known as acetylene, is an organic chemical compound with the chemical formula C2H2. Since the entire chemical composition only features hydrogen and carbon atoms, this compound is a hydrocarbon. It is a colorless gaseous chemical compound and the simplest alkyne, i.e., an unsaturated hydrocarbon with a triple C-C Bond. Pure ethyne is known to be highly unstable. Therefore, it is common for ethyne to be handled in a solution. Generally, acetylene is used as a fuel and a chemical building block.
Structure
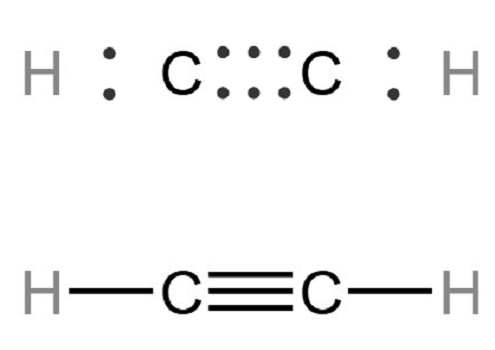
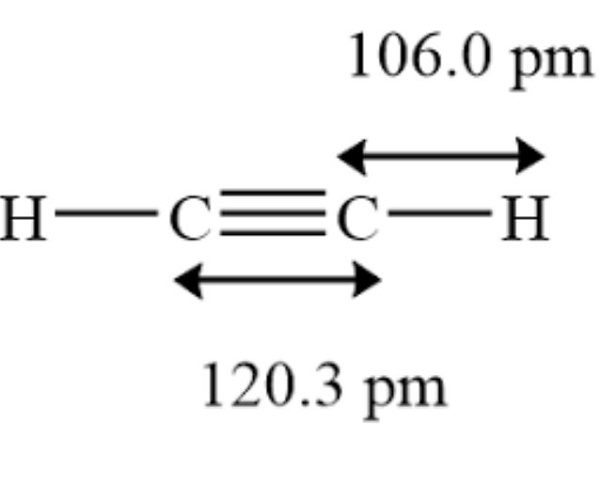
The ethyne molecule features a triple bond between two carbon atoms, each singly bonded to another hydrogen atom. It can be noted that all four atoms are placed in a single line, with the hydrogen atoms at the corners and carbon atoms with a triple bond between them in the center. In addition, the bond angle is 180°. It can also be noted that the bond length of the carbon-hydrogen bond in the ethyne molecule is roughly equal to 106 picometres, whereas the carbon-carbon bond length in the molecule is approximately equal to 120.3 picometres.
C2H2 is nonpolar in nature. The polarity of any molecule depends on the following factors: The difference in electronegativities of atoms. The shape of the molecule. Dipole moment. Distribution of charges
The electronegativity difference between carbon and hydrogen is 0.35, less than the minimum required 0.4. Moreover, as the difference in electronegativities is not that high, this molecule will have nearly no to zero dipole moment. Furthermore, it has a linear molecular structure, and the nature of C-H bonds is nonpolar covalent. This makes the complete C2H2 molecule a nonpolar molecule with a net zero dipole moment. Moreover, as there is no net dipole moment, there are no poles in this molecule. The charges in the molecule are evenly distributed, and there are no positively charged or negatively charged areas in the molecule. This even distribution of the charges also makes this molecule a nonpolar molecule.