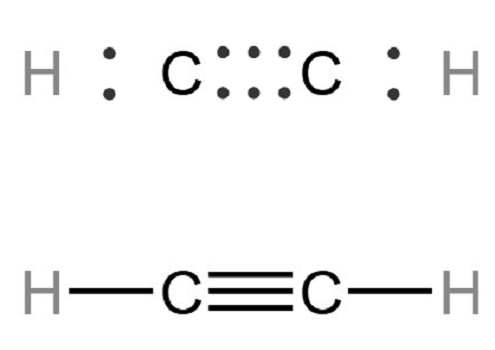
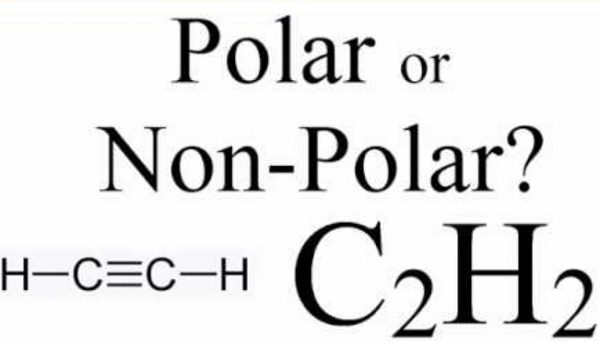Is acetylene dissolved in water more stable than gaseous acetylene?
Description
Acetylene, also known as aldehyde, ethyne, and ethine, is a colorless gas with ether odor at room temperature and atmospheric pressure. Acetylene contains relatively active unsaturated triple bonds and can react with many substances.
Uses
From acetylene, thousands of organic chemical products can be synthesized, making acetylene a basic raw material for synthetic plastics, synthetic rubber, synthetic fibers, pharmaceuticals, pesticides, dyes, resins, and solvents. Even acetylene was once known as the “mother of the organic synthesis industry.” Compared with ethylene and propylene, acetylene used as raw material has the advantages of short and mature processes. Therefore, it still occupies a certain proportion in the synthesis of many products, especially in fine chemicals. Acetylene has advantages in preparing fine chemicals, and many fine chemicals can only be manufactured by acetylene.
chemical property
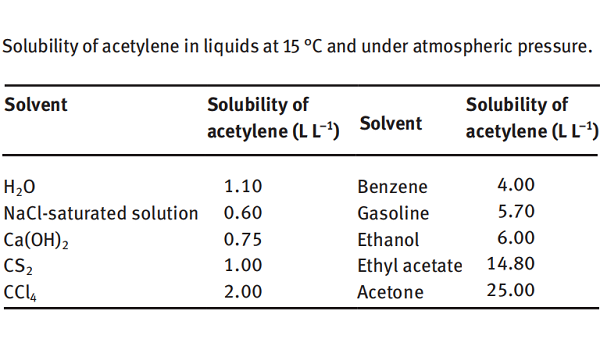
Acetylene is soluble in water and ethanol and very soluble in acetone. The solubility of acetylene in some liquids is shown below. It can be seen that 1 L of water can dissolve 1.1 L of acetylene at 15 °C and under atmospheric pressure. In comparison, 1 L of acetone can dissolve 25 L of acetylene under the same conditions. Under a pressure of 1.2 MPa, 1 L of acetone can dissolve 300 L of acetylene. Since acetylene has a very large solubility in acetone, acetylene is pressed into a special cylinder containing acetone and porous material (such as activated carbon) under a pressure of 1.0–1.2 MPa, known as “dissolved acetylene.” This dissolved acetylene is safe to use and convenient for transportation and storage. Other excellent solvents for acetylene include liquid ammonia, N, Ndimethylformamide, N-methylpyrrolidone, methylal, dimethoxyethane, dioxane, tetraethylene glycol dimethyl ether and 2-pyrrolidone.
When acetylene comes in contact with water, it can form hydrated crystals composed of one molecule of acetylene and six molecules of water at a specific pressure and temperature, like ice and snow. The highest temperature of hydrated crystals is 15 °C. Above this temperature, the hydrated crystals cannot exist regardless of the pressure. The presence of hydrated crystals in acetylene may cause blockage in the acetylene pipeline or may cause danger due to static electricity generated by the friction between the acetylene gas and the hydrated crystals.

Acetylene gas is relatively unstable and has the risk of decomposition and explosion under high temperatures and pressure. The explosion risk of wet acetylene is less than that of dry acetylene, decreasing further with the increase in humidity. When acetylene is dissolved in a liquid, its molecules are separated by solvent molecules, and it is stable and safe to handle at high temperature and pressure as long as it remains in the liquid phase. The explosion limit of acetylene and oxygen mixture is 2.3–93%, and the explosion limit of acetylene in air is 2.3–81%. The temperature of oxyacetylene flame produced by acetylene combustion can reach more than 2,800 °C, which can be used to cut and weld metals. When acetylene comes in contact with salts of copper, silver, and mercury in aqueous solution, the precipitates of the corresponding metal acetylene are formed, which are explosive and easy to explode when impacted or heated.