What is the Lewis structure for acetylene?
Introduce
Acetylene is the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds, called the acetylenic series, or alkynes. It is a colorless, flammable gas with the chemical formula C2H2. This compound is widely used as a fuel in oxyacetylene welding and the cutting of metals and as raw material in the synthesis of many organic chemicals and plastics.
The hottest and most efficient fuel gas, acetylene, provides high productivity levels thanks to good localized heating with minimal thermal waste. It also requires the least amount of oxygen to ensure complete combustion. This flammable, colorless gas is lighter than air, so it does not accumulate at low levels, where it could cause a potential hazard. It is generally supplied dissolved in acetone or DMF.
Lewis Structure
Lewis Structure of any molecule helps to know the arrangement of all atoms, their valence electrons, and the bond formation in the molecule. The electrons that form bonds are called bonding pairs of electrons, while those that do not take part in any bond formation are called lone pairs or non-bonding pairs of electrons. The Lewis Structure of C2H2 is shown below:
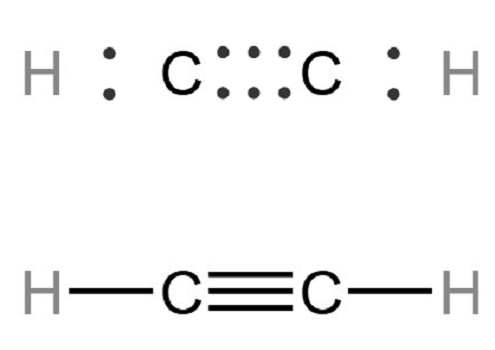
The Lewis structure of acetylene (C2H2) is made up of two carbon (C) atoms and two Hydrogen (H) atoms. An anyone carbon atom can be considered a central carbon. In this structure, in addition to a complete duplet of the outer H-atoms, the two C-atoms at the center also have a complete octet with 1 mutually shared triple covalent bond and a C-H single bond at each terminal. Regarding this central C-atom, there are 2 electron-density regions around it. Both electron density regions are comprised of bond pairs. Thus, there is no lone pair of electrons on this central C-atom in the C2H2 Lewis dot structure.
Hybridization of C2H2
The hybridization of carbon atoms in the acetylene (C2H2) molecule is sp, whereas the hydrogen atoms have unhybridized 1s atomic orbitals. In the case of sp hybridization, the s orbital of the central atom only binds with one of its p orbitals.