About the polarity of NF3
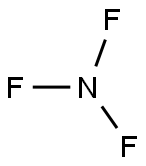
Nitrogen trifluoride is a chemical compound with chemical formula NF3. It exists as a colorless gas having a musty smell at room temperature. It is non-flammable in nature. Many students may have a question regarding whether NF3 is polar or not.
Is NF3 Polar or Nonpolar?
So, is NF3 Polar or Nonpolar? NF3 is polar in nature due to the presence of lone pair on nitrogen atom causing a distorted shape of NF3 molecule and the difference between the electronegativity of fluorine(3.98) and nitrogen(3.04) causes polarity in N-F bonds and result in a non zero dipole moment of the entire molecule.
Nitrogen trifluoride exists in the form of colorless gas with musty odor at the standard conditions of temperature and pressure.
It was first synthesized by the scientist Otto Ruff in the year 1903 by the process of electrolysis of a molten mixture of hydrogen fluoride and ammonium fluoride.
It is known to be lesser reactive than other halides of nitrogen like nitrogen trichloride, nitrogen tribromide, etc.
It is manufactured directly by reacting nitrogen with fluorine and commercially it is supplied in pressurized cylinders.
Nitrogen trifluoride gas is considered as a strong greenhouse gas (causing global warming).
The structure of nitrogen trifluoride
The molecule of nitrogen trifluoride is made up of 1 nitrogen atom and 3 fluorine atoms. The nitrogen atom is a central atom surrounded by three fluorine atoms.
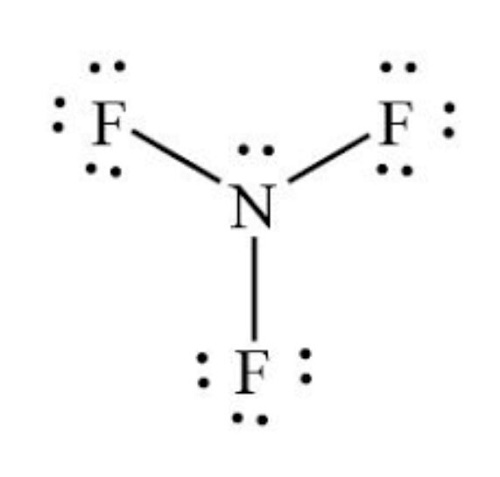
The molecule of NF3 consists of a total of 8 valence electrons. Nitrogen atom contains 5 electrons in its outermost shell and requires 3 electrons to complete its octet.
And, Fluorine has 7 valence electrons in its outermost shell and requires a single electron to complete its octet.
Accordingly, all three fluorine atoms share one electron of the nitrogen atom to get stabilize leaving behind one lone pair on the nitrogen atom.
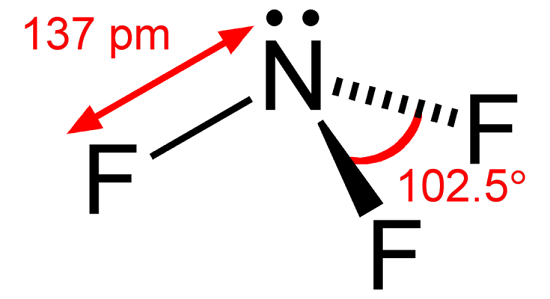
The shape of the Nitrogen trifluoride is formed as trigonal pyramidal similar to the shape of the ammonia molecule(NH3).