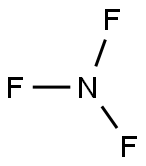
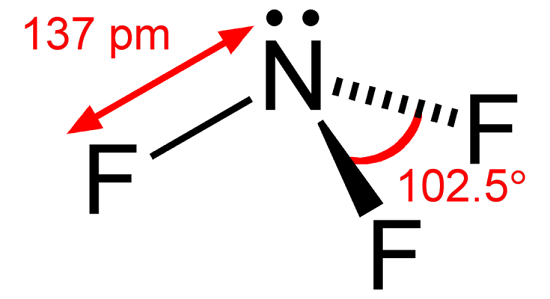
What is the Lewis structure of nitrogen trifluoride
What is NF3?
Nitrogen trifluoride (NF3) is a colorless, nonflammable gas with routine usage in the microelectronics industry. It is an essential molecule in plasma science as an efficient fluorine source in manufacturing massive-scale integrated circuits. Although NF3 is indispensable in the electronics industry, it is a significant greenhouse gas, and its heat storage capacity is 17,000 times that of carbon dioxide. The molecule is a hazardous greenhouse gas that can persist in the atmosphere for 740 years. In 2008, it was included in the list of controlled gases under the United Nations Framework Convention on Climate Change[1-3].
Steps of drawing Lewis structure of NF3
Step 1: Total number of electrons of the valance shells of NF3
Fluorine is a group VIIA element and has seven electrons in its last shell (valence shell). Nitrogen is a group VA element in the periodic table and contains five electrons in its last shell. Hence, Total valence electrons in NF3 molecule = valence electrons given by one nitrogen atom + valence electrons given by three fluorine atoms = 5 + 7(3) = 26.
Step 2: Select the central atom
It is essential to consider both the ability to have a greater valence and to be the most electropositive element. Nitrogen, with a maximum valence of 5, has a higher valence than fluorine, which has a maximum valence of 1. Nitrogen has a lower electronegativity value (2.1) than fluorine (4.0). So, Nitrogen was placed at the center, and fluorine was spaced evenly around it.
Step 3: Connect each atom by putting an electron pair between them
In the NF3 molecule, put the electron pairs between the nitrogen atom (N) and fluorine atoms (F). This indicates that Nitrogen and fluorine are chemically bonded with each other in an NF3 molecule.
Step 4: Placed remaining valence electrons starting from the outer atom first
We have found the center atom and sketch of the NF3 molecule. Since there are already three N-F bonds in the molecule, ten (13–3) electron pairs are left to be marked on the atoms. Typically, the remaining electron pairs are marked on the outside atoms first, which, in this case, are the fluorine atoms. Therefore, three lone pairs will be marked on each fluorine atom, totaling 9 electron pairs. Finally, the remaining lone pair can be marked on the nitrogen atom.
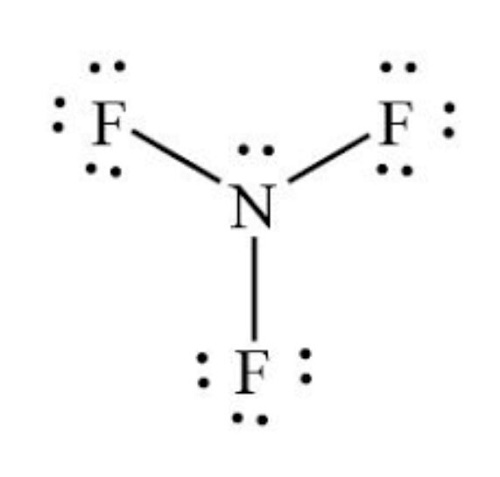
Step 5: Check the stability of lewis structure
The stability of Lewis's structure can be checked by using the concept of a formal charge.
Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons
For nitrogen atom-atom: Formal charge=5-2-6/2=0
For fluorine atom: Formal charge=7-6-2/2=0
Since the overall formal charge is zero, the above Lewis structure of NF3 is most appropriate, reliable, and stable.
References
[1] Zhu, Ma Lin Fan . "Plasma-relevant fast electron impact study of nitrogen trifluoride." Plasma Sources Science & Technology 29.8(2020).
[2] Jingyan Zheng. "Spectroscopic characteristics and dissociation of nitrogen trifluoride under external electric fields: Theoretical study." Open Physics 20 1 (2022): 1203–1212.
[3] Ray F. Weiss. "Nitrogen trifluoride in the global atmosphere." Geophysical Research Letters 35 20 (2008).