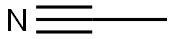Is acetonitrile a highly polar solvent?
Overview
Acetonitrile is a volatile organic molecule composed of carbon, hydrogen, and nitrogen atoms. It is also called Cyanomethane or Methanecarbonitrile. The chemical formula of Acetonitrile is C2H3N. It is the simplest organic nitrile produced as a byproduct in acrylonitrile synthesis. Acetonitrile is found in the environment, mainly in the exhaust of automobiles and in the air in industrial sites[1].
Structure
Acetonitrile is an organic molecule whose functional group is the nitrile group, composed of a single carbon atom sharing three pairs of electrons with a nitrogen atom. The acetonitrile structure is perfectly linear; the bond angles between the terminal carbon, the central carbon, and the nitrogen are all 180. The bond angle between the central C and the N atoms is 180 due to the triple bonds. The bond angle between the terminal C and the central C is also 180, and this angle is perfect for minimizing the effects of electron repulsion. Bonds are negatively charged electrons and like repels like, which is why covalent bonds prefer to be as far apart as possible.
Polar or nonpolar
As shown in the figure below, it is a highly polar, volatile organic compound used as a solvent in many industrial applications. The Polarity Index of this compound is 5.8. In comparison, the Polarity Index of Pentane is 0, and Wter's Polarity Index is 10.2.
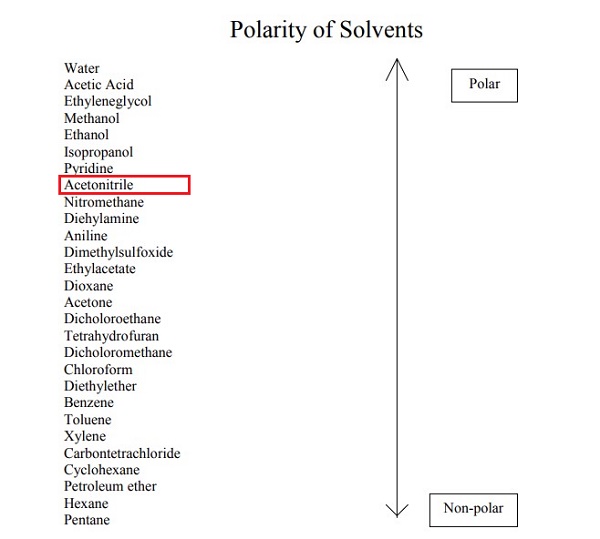
The sharing of electrons in the polar molecule is unequal. The nitrogen atom is the main contributor to polarity; the electronegativity difference between the nitrogen and the carbon is the reason for the acetonitrile polarity. The electronegativity of the nitrogen atom is much higher than that of the carbon, which is why it draws the electrons it is sharing with C closer. This is how the uneven distribution of electrons is induced. The nitrogen atom has a partial negative charge, while its adjacent carbon has a partial positive charge.
Polarity and uses
In terms of polarity, acetonitrile shows several unexpected physicochemical properties that may strongly affect the final results of analytical protocols applied in detection and separation science[2]. In particular, pure acetonitrile, as well binary mixtures of it with water within a narrow concentration range from 90 to 100% (v/v), are relatively nonpolar and can strongly influence the elution power of a chromatographic mobile phase and the efficiency with which components of interest can be extracted from complex samples. The atypical freezing-point curve of the ACN–water mixture is associated with a solvent polarity change in the concentration range of 90–100% ACN. For example, the retention of testosterone and its derivative, testosterone isobutyrate, across a range of ACN-in-water concentrations can be traced using planar chromatography. The more polar testosterone is eluted as the first steroid for ACN concentrations of up to 90% (v/v). Within this range, the less polar testosterone isobutyrate elutes before testosterone. This observation directly confirms that the polarity of a mobile phase consisting mostly of ACN changes significantly as the ACN concentration approaches 100%. It can be concluded that pure acetonitrile with a low water content may function as a relatively nonpolar liquid and that the polarity of ACN can easily be altered by adding a small volume of water.
References
[1] H. Robles. "Acetonitrile." Encyclopedia of Toxicology (Third Edition) (2014): 40-42.
[2] P. K. Zarzycki. "Acetonitrile, the polarity chameleon." Analytical and Bioanalytical Chemistry 397 3 (2010): 905–908.
You may like
Related articles And Qustion
See also
Lastest Price from Acetonitrile manufacturers

US $0.00/tons2025-06-16
- CAS:
- 75-05-8
- Min. Order:
- 18tons
- Purity:
- 99.99%
- Supply Ability:
- 5000ton

US $1.00/KG2025-05-14
- CAS:
- 75-05-8
- Min. Order:
- 1000KG
- Purity:
- 99.99
- Supply Ability:
- QTY LARGE