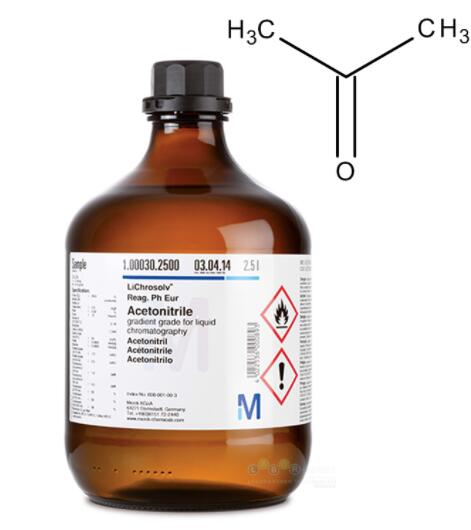
What is Acetonitrile?
Acetonitrile is a liquid with an etherlike odor. It is a highly polar, volatile solvent used in many different industrial applications. It is widely used in the pharmaceutical, photographic, chemical, and analytical industries. It is useful as an industrial solvent for the separation of olefins, polymers, spinning fibers, and plastics. Other uses include the extraction and refining of copper and by-product ammonium sulfate; used for dyeing textiles and in coating compositions; used as a stabilizer for chlorinated solvents; manufacture of perfumes and cosmetics; and as a general reagent in a wide variety of chemical processes.
Uses
Acetonitrile is used in the chemical industry as an intermediary in the synthesis of several chemicals and products such as acetophene, thiamine, acetamidine, alpha-naphthaleneacetic acid, nitrogen-containing compounds, acrylic fibers, nitrile rubber, pesticides, pharmaceuticals, perfumes, and lithium batteries. It is also used as a polar solvent for both organic and inorganic compounds and in nonaqueous titrations.
Mechanism of Toxicity
If released to ambient air, acetonitrile will remain in the vapor phase where it will be degraded through reaction with photochemically produced hydroxyl radicals. The half-life of acetonitrile in ambient air has been estimated to be about 620 days. If released to soil, acetonitrile is expected to volatilize rapidly. Biodegradation in soil is not expected to be a major degradation pathway. If released to water, acetonitrile is not likely to adsorb to soil and sediment particles. Acetonitrile is expected to be removed from water bodies through volatilization, as the chemical hydrolysis and bioaccumulation potential for this chemical are low.
Environmental Fate
Acetonitrile is slowly metabolized by cytochrome P450 in the liver to produce hydrogen cyanide. Toxicity is produced by the combined effect of circulating acetonitrile and cyanide. Cyanide exerts its toxicological effects by disrupting oxygen utilization at the cellular level. The disruption results in decreased oxygen utilization by body tissues and lactic acidosis.
You may like
Related articles And Qustion
See also
Lastest Price from Acetonitrile manufacturers

US $0.00/tons2025-06-16
- CAS:
- 75-05-8
- Min. Order:
- 18tons
- Purity:
- 99.99%
- Supply Ability:
- 5000ton

US $1.00/KG2025-05-14
- CAS:
- 75-05-8
- Min. Order:
- 1000KG
- Purity:
- 99.99
- Supply Ability:
- QTY LARGE