Betulin: Properties, Application, pharmacokinetics, Synthesis etc.
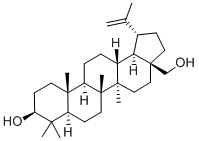
Betulin (CAS No: 473-98-3) was first observed by Lowitz in 1788, from the sublimation products of birch bark. Betulinic acid (3β, hydroxyl-lup-20(29)-en-28-oic acid) (BA) is a triterpenoid of lupane-structured pentacyclic triterpene. It can be isolated from many plants, mainly the bark of Betulaceae family. It can also be synthesized by the oxidation of betulin (lup-20(29)-ene-3β, 28-diol) (B). BA and B are mainly isolated from the various parts of Betulaceae, Platanaceae, Dilleniaceae, Rhamnaceae, Rosaceae, Fagaceae families. Betulin and BA are pentacyclic triterpene natural products that are observed as secondary metabolites in more than 200 different types of plants. Birch bark is the main source of B and BA and is divided into two clearly external and internal distinguished parts. The outer part of the birch bark is especially rich in the extractives. The bark of white birch trees contains different levels of B (10-35 %) depending on the age of the tree, site of ground and conditions, birch species and some other factors. Along with these two extracts of the bark of these species, other B derivatives like betulinic aldehyde, methyl ester of betulinic acid, betulonic aldehyde, betulonic acid (BE), and triterpenes of oleanane and ursane series could also be found. The extracts of the internal part of the bark are the source of phenolic compounds although small amounts of B can also be found.
Properties
Low water solubility and lipophilic characteristic of B and most of the B derivatives have a consequential role in interpreting the bioactivity assay results of cell cultures. Limited solubility of B derivatives in aqueous media has become one of the important challenges associated with the development of B analogs as therapeutic agents (Ali-Seyed et al., 2016; Dehaen et al., 2011; Jonnalagadda et al., 2013; Periasamy et al., 2014; Tolstikov et al., 2005; Yogeeswari and Sriram, 2005; Zhang et al., 2015a). Due to the high order of BA safety, lots of structural modifications have been investigated to improve its potency and efficacy as a result of improvement of its solubility profile.[3]
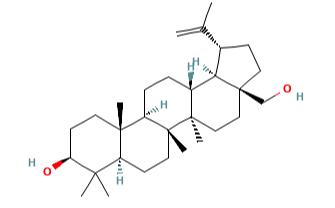
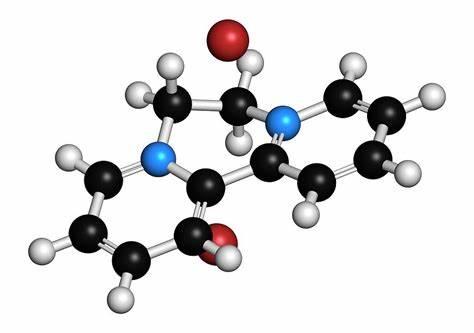
Figure 1 the molecular formula of Betulin
Application and pharmacokinetics
Betulin (B) and Betulinic acid (BA) are natural pentacyclic lupane-structure triterpenoids which possess a wide range of pharmacological activities. Recent evidence indicates that B and BA have several properties useful for the treatment of metabolic disorders, infectious diseases, cardiovascular disorders, and neurological disorders. In the current review, we discuss B and BA structures and derivatives and then comprehensively explain their pharmacological effects in relation to various diseases.
Sterol regulatory element-binding proteins (SREBPs) are major transcription factors activating the expression of genes involved in biosynthesis of cholesterol, fatty acid and triglyceride. The study identified a small molecule, betulin, that specifically inhibited the maturation of SREBP by inducing interaction of SREBP cleavage activating protein (SCAP) and Insig. Inhibition of SREBP by betulin decreased the biosynthesis of cholesterol and fatty acid. In vivo, betulin ameliorated diet-induced obesity, decreased the lipid contents in serum and tissues, and increased insulin sensitivity. Furthermore, betulin reduced the size and improved the stability of atherosclerotic plaques. It demonstrates that inhibition SREBP pathway can be employed as a therapeutic strategy to treat metabolic diseases including type II diabetes and atherosclerosis. Betulin, which is abundant in birch bark, could be a leading compound for development of drugs for hyperlipidemia.[1]
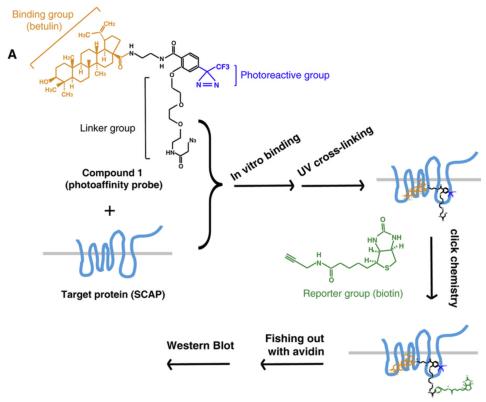
Figure 2 Betulin Binds to SCAP
3.Orphan Drug Designation for the Indication[2]:
Epidermolysis BullosaBoth the EMA, in 2011, and the FDA, in 2014, issued an orphan drug designation for the active ingredient betulin for the indication epidermolysis bullosa. In this rare disease, which is also known as butterfly disease, the skin lacks certain anchor proteins due to hereditary factors, with the result that even slight mechanical stress results in detachment of the skin, with open wounds or blisters. Case reports and a phase II study on the acceleration of reepithelialisation revealed birch bark extract to be beneficial forwound healing. Verification by means of a substantiating phase III study was initiated in April 2017. An interim efficacy analysis at the end of 2018 recommended that the trial should contin-ue with an increase of 48 patients to a total of 230. Results are expected in H2 2019.
Mode of Action: Acceleration in All 3 Phases:
of Wound HealingAs already mentioned above, studies on the pharmacological effect of betulins were carried out during the development years, from 2006 to 2013. In collaboration with the research groups of Professor Schempp and Professor Merfort in Freiburg, as well as Professor Brandner in Hamburg, important evidence of the effect of betulins and the dry extract (TE) was obtained for the different phases of wound healing. Wound healing takes place in three stages: the process begins with the inflammatory phase, in which mediators are released to attract macrophages, phagocytes and granulocytes into the wound in order to clean the wound. In the second phase, the inflammation is resolved, and the skin cells multiply and migrate in order to close the wound. The third and final phase is the longest lasting. The skin cells differentiate, mature and remodel the wound.
Synthesis
Isopentenyl pyrophosphate oligomers comprise one of the extended assortments of plant-derived natural product with over 30,000 members. These compounds are known as "Terpene" which originates from turpentine, the so-called “resin of pine trees”- a viscous pleasantly smelling balsam flowing upon carving the bark and the new wood of plenty of pine trees parts. Three adjoining terpene units form a class of chemical compound known as Triterpenes. Usually, triterpenes occur in their free form or as glycosides. Abundant numbers of these compounds adopt tetracyclic and pentacyclic structures. Gammaceranes, hopanes, lupanes, oleananes, ursanes are a few of pentacyclic triterpenes subclasses, in which are allocated based on the chemical structures of their carbon skeletons. Triterpenoids are functionalized triterpenes, which can be synthesized through the cyclization of the squalene intermediate with six isoprene units from isopentenyl pyrophosphate. Betulin (lup-20(29)-ene-3b, 28 diol) (B) and betulinic acid (BA) (3β-hydroxy-lup-20(29)-en-28-oic acid) are lupane structured pentacyclic triterpenoids. The structures of B and BA are shown in Figure 2. The existence of five-membered E ring and isopropylidene group in the molecule are common structural features of the lupane skeleton.[3]
Toxicological and Safety
The conversion of betulonate as a precursor to various derivatives like hemisuccinate analog, succinic acid hemiester, C-2-hydroxybetulonic acid ester, 2,2-difluoro and 2,2-dibromo derivatives was achieved based on the halogenetion/trifluoromethylation method. The 2,2- difluoro and 2,2-dibromo derivatives exhibited promising cytotoxicity against Leukemia CEM cancer lines.
Handling and storage
Powder grayish white or beige may be harmful if swallowed., May damage (eyes) organs. Consult a doctor., Show this safety technical instruction to the doctor on site. If inhaled, remove the patient to fresh air., If breathing stops, perform artificial respiration., Consult a doctor. Rinse with soap and plenty of water., Send the patient to the hospital immediately., Consult a doctor. Wash eyes with water carefully. Never feed anything to an unconscious person., Rinse your mouth with water., Consult a doctor.
Fire extinguishing methods and extinguishing agents are water mist, alcohol resistant foam, dry powder or carbon dioxide fire extinguishing.
Use personal protective equipment. Avoid dust generation. Avoid breathing vapors, aerosols or gases. Ensure adequate ventilation. Evacuate personnel to a safe area. Avoid inhaling dust.
References
1, "Inhibition of SREBP by a Small Molecule, Betulin, Improves Hyperlipidemia and Insulin Resistance and Reduces Atherosclerotic Plaques,".
2 Scheffler A., "The Wound Healing Properties of Betulin from Birch Bark from Bench to Bedside," Planta medica, Vol.85, No.7(2019), pp.524-527.
3 , "etulin and its Derivatives as Novel Compounds with Different Pharmacological Effects,".
You may like
Related articles And Qustion
Lastest Price from Betulin manufacturers

US $0.00/KG2025-08-27
- CAS:
- 473-98-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month

US $5.00-0.50/KG2025-05-08
- CAS:
- 473-98-3
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS