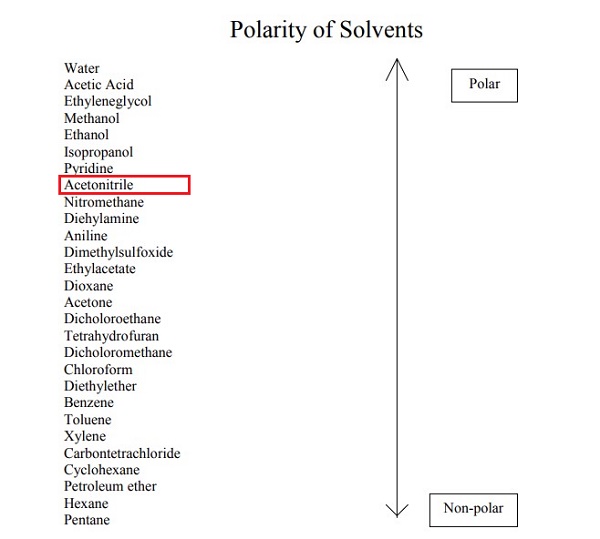
Effect of Additives on Acetonitrile Polarity
Acetonitrile (CHCN, ACN) is a relatively nontoxic, highly volatile and aprotic polar organic solvent. ACN plays a key role as an extraction and deproteinization medium for a variety of pre-purification and concentration techniques using liquid-liquid extraction, solid-phase extraction (SPE) or microextraction (SPME) protocols. In terms of polarity, acetonitrile shows a number of unexpected physicochemical properties that may strongly affect the final results of analytical protocols applied in detection and separation science. Strong hydrogen bonding and the creation of low-polar self-associated forms of acetonitrile affect a number of physical and chemical properties of acetonitrile-water mixtures. The relatively low polarity of pure ACN can easily be disrupted by the presence of a small amount of water, which is usually present in either the raw biological sample or the ACN itself[1]. The polarity of acetonitrile is easily affected by additives. Examples will be given below.
CO2-Expanded Acetonitrile[2]
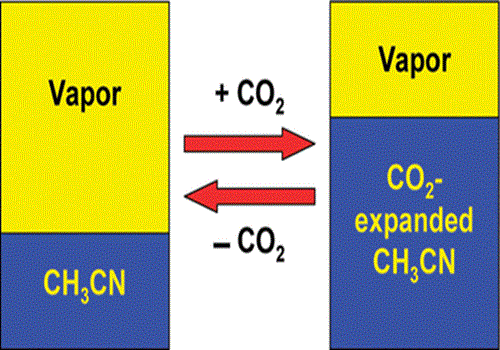
Many processes that use highly tunable gas-expanded liquids (GXLs) rely on the fact that CO2 addition can greatly affect the polarity of the solvent. Authors have examined several measures of bulk and local polarity in CO2-expanded acetonitrile to enable more effective exploitation of these polarity changes. The rate of the nucleophilic substitution reaction of tributylamine with methyl p-nitrobenzenesulfonate has been analyzed as a function of solvent composition by using in situ high-pressure UV/vis spectroscopy. Authors have also measured solvatochromic properties including the Kamlet−Taft π* parameter and Kosower's Z-value. Then correlate these local polarity-based kinetic and solvatochromic measures to develop a better understanding of these property changes as a function of bulk and local solvent composition. The data suggest that local composition enhancement in CO2-expanded acetonitrile has a significant impact on the reaction kinetics.
Imidazolium Ionic Liquids as Additives[3]
The use of specific additives in order to change some physical-chemical properties of different organic compounds is a relevant research topic. The possibility of modifying the polarities of organic solvents can be an important tool to solve certain separation processes. Authors have studied the influence in acetonitrile polarity by the addition of small quantities of ionic liquids based on imidazolium cations. The use of ionic liquids as additives can be an efficient mechanism for the modification of organic solvent polarities as well as to open new perspectives in chemical sciences.
Reference
[1] Acetonitrile, the polarity chameleon. Analytical and Bioanalytical Chemistry 397 (3) 905-908.
[2] Local Polarity in CO2-Expanded Acetonitrile: A Nucleophilic Substitution Reaction and Solvatochromic Probes. J. Org. Chem. 2008, 73, 9, 3364–3368.
[3] Studies of the Influence in Acetonitrile Polarity Using Imidazolium Ionic Liquids as Additives. J. Chem. Eng. Data 2013, 58, 6, 1449–1453.
You may like
Related articles And Qustion
See also
Lastest Price from Acetonitrile manufacturers

US $0.00/tons2025-06-16
- CAS:
- 75-05-8
- Min. Order:
- 18tons
- Purity:
- 99.99%
- Supply Ability:
- 5000ton

US $1.00/KG2025-05-14
- CAS:
- 75-05-8
- Min. Order:
- 1000KG
- Purity:
- 99.99
- Supply Ability:
- QTY LARGE