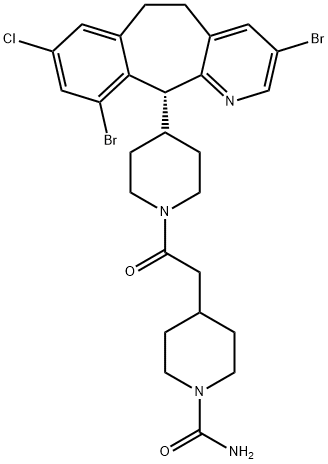
How is Lonafarnib synthesised?
Synthesis_Synthesis of Lonafarnib
Lonafarnib was synthesised starting from the bromination of chloroanthranilic acid and using the lonafarnib precursor tricycle methodology based on a Grignard-induced intramolecular cyclisation reaction. The specific synthesis steps are as follows:
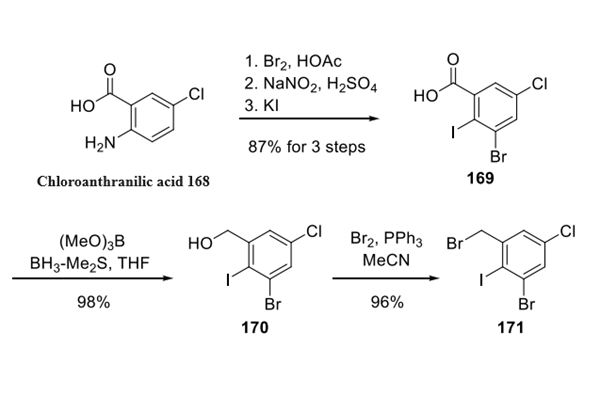
Step 1: Preparation of Lonafarnib-Related Aryl Bromide
The synthesis began with bromination of chloroanthranilic acid 168, followed by nitration, diazotization, and treatment with potassium iodide to afford iodide 169. This sequence was followed by carboxylate reduction and bromination to give rise to aryl bromide 171.
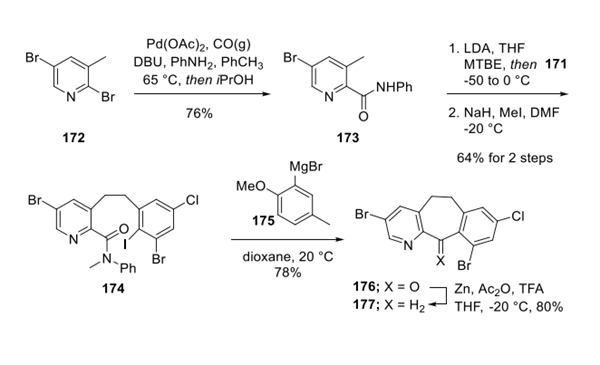
Step 2: Preparation of Lonafarnib Tricycle
Separately, carbonylation of 2-bromopyridine 172 in the presence of aniline gave rise to amide 173, which was then doubly deprotonated with LDA and quenched with benzyl bromide 171. Methylation using NaH and MeI furnished the ethylene biaryl subunit 174. Exposure to Grignard reagent 175 facilitated transmetalation and an intramolecular ring closing reaction to form tricyclic ketone 176, which wasreduced using modifiedClemmensen conditions to arrive at ketone 177.
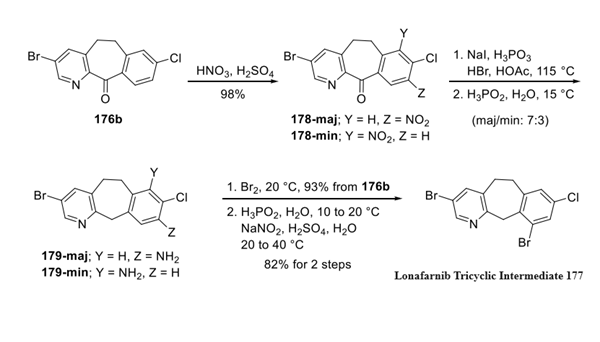
Step 3: Conversion of 176b to Lonafarnib Tricyclic Intermediate
As disclosed in a 2004 Schering-Plough Research Institute patent, des-bromo 176b underwent a nitration to give a mixture of regioisomeric nitration products in a 7:3 ratio with the major product being the C-9 nitroarene (178-maj). This mixture was not separated but instead subjected to reduction conditions specifically designed for these substrates, which employed catalytic iodide in acid followed by a workup with aqueous phosphoric acid. This served to both remove the ketone functionality and reduce the nitro groups, producing the corresponding mixture of anilines 179. Interestingly, bromination of this mixture resulted in substitution exclusively at the C-8 position regardless of aniline regiochemistry. This high-yielding step was followed by diazotization of the anilines, which furnished the key intermediate 177 in good yield.
Step 4: Preparation of Lonafarnib
Removal of the doubly benzylic proton within tricycle 177 in the presence of quinine and mesylate 180 led to the formation of intermediate 181 in excellent conversion and 95%ee. The authors attributed the remarkable selectivity to three points of interaction with quinine, which take place between the alkoxy group, to chelation of the bridgehead nitrogen of quinine and lithiated 177, and from the π-stacking between the quinoline moiety and the pyridine ring, which was further enhanced by the electron-donating methoxy group of quinine.
In addition, it was found that a single equivalent of water had a pronounced improvement on selectivity and consistently provided 95% ee. The Boc group wasthen removed in situ under acidic conditions, and 181 was crystallized as an N-acetyl-L-phenylalanine salt 182 in 80% yield from 177, further resolving the ee to 99%. Lastly, NBoc piperidine acetic acid 183 was coupled to 182 using standard amide bond formation conditions. Subsequent Boc deprotection and urea formation using NMP and urea ultimately produced lonafarnib.