Introduction of Naphthalene
General description
Eugenol (C10H12O2) (CAS No: 97-53-0) is a volatile phenolic constituent of clove essential oil obtained from Eugenia caryophyllata buds and leaves, mainly harvested in Indonesia, India and Madagascar, Figure 1. The name supposedly is derived from the scientific name for clove E. caryophyllata tree which has large leaves and flower buds which turn to red color when they are ready for collection [1-2]. Eugenol is the main extracted constituent (70-90%) of cloves and is responsible for clove aroma [1]. Eugenol is pale yellow oil with a spicy aroma with the molecular weight of 164.2 g/mol. This molecule is a weak acid which is soluble in organic solvents and specially extracted from clove oil, nutmeg, cinnamon, basil and bay leaf. There are different types of essential oil extracted from parts of clove. The oil derived from the flower buds of clove mainly consists of eugenol (60-90%), eugenyl acetate, caryophyllene and other substances, whereas oil derived from the leaves of the clove tree consists of eugenol (82-88%) and very little eugenyl acetate, and other minor constituents. The oil derived from the twigs of cloves consists of 90-95% of eugenol. Eugenol also can be produced synthetically by the allylation of guaiacol with allylchloride [3-4].
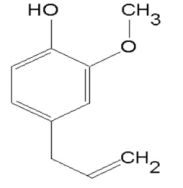
Figure 1. Chemical structure of eugenol (C10H12O2)
Applications of eugenol
Since ancient times, clove oil has been used as an antimicrobial, antiseptic and antispasmodic in Chinese traditional medicine. Nowadays, there is also a wide range of use of eugenol for several purposes such as household products, fragrance in soaps and cosmetics, skin care products, flavoring substance for food, dental and pharmaceutical products [1]. Eugenol causes an enhancement in skin penetration of diverse drugs. It is also used in agricultural applications to protect foods from microorganisms such as Listeria monocytogenes and Lactobacillus during storage, as a pesticide and fumigant [5].The US Food and Drug Administration approved the use of clove oil as a flavoring substance in the food industry, as a fragrance in cosmetics industry and in dentistry as a natural analgesic and antiseptic [6]. There are relatively few human studies about the pharmacokinetic and effects of eugenol. Animal studies have suggested that after inhalation of the smoke of clove cigarettes, minor amounts of eugenol may be absorbed from the lung. Also some in vitro studies indicated that eugenol undergoes biotransformation in hepatocytes [7].
pharmacokinetics of eugenol
Eugenol is well-known for its antibacterial properties. For this reason, it has been extensively used for oral and dental care. It reduces local pain and has disinfecting activities. In dental medicine, amorphous chelate compounds made from eugenol in conjunction with zinc oxide are used for covering the pulp indirectly. It is also utilized to fill root canals in liquid form in particular pastes. Occasionally, eugenol is put on the gums for numbing prior to dentures being introduced [8-10]. Eugenol is usually utilized in medications, cosmetics, food, and as a local analgesic and antiseptic at low concentrations and has numerous actions (Figure 2). Furthermore, eugenol is a general component that is found in a variety of goods around the house, like soaps, fragrances, and skincare products. Additionally, it is utilized as a pesticide, fumigant, and preservative to keep foods safe from microbes. According to the Joint FAO/WHO Expert Committee on Food Additives (JECFA), the maximum daily dose of eugenol for humans is 2.5 mg/kg body weight [11-13]. Eugenol is utilized to treat gastrointestinal and respiratory contagions. Furthermore, it comprises many medicines that have been suggested for the treatment of upper respiratory mucosa inflammation and cold prevention. These medicines are generally administered as inhalation and aerosol remedies, such as Aromatol, Amol, or Olbas [14]. Eugenol has applications in food manufacturing and agriculture as a result of its diverse activities, such as antimicrobial. Its valuable influence is linked with low doses of efficient action, which is a significant benefit. Furthermore, eugenol is efficient against numerous foodborne pathogens [15]. Eugenol is also utilized as a biocontrol in agriculture due to its potential to decrease Salmonella infection of organic products via inhibition of its growth in soil. Furthermore, its antifungal characteristics are used to preserve fruits like apples, strawberries, and peaches, as well as their juices, from hazardous microbes [14]. Although excessive amounts of eugenol can cause harmful effects, a concentration of 2.5 mg/kg body weight is commonly considered harmless. Due to its use in dentistry, via lung exposure to LPS. Therefore, eugenol can protect against the damage produced via oxidative stress and can also be employed as an anti-inflammatory drug [15-16]. Although eugenol is recognized to possess antioxidant and anti-inflammatory activities at small dosages, at greater concentrations, it can have a pro-oxidative influence, causing the production of free radicals. Furthermore, DNA disintegrated in normal human fibroblast cells can be raised under the influence of clove oil at high concentrations, according to several studies [17].
An investigation of male and female healthy volunteers demonstrated that eugenol was absorbed and metabolized after oral administration rapidly. It was almost completely excreted in the urine within 24 hours and the urine contained conjugates of eugenol. Results revealed that only less than 1% of administrated dose was excreted as non-metabolized in urine. Analysis of urine also demonstrated that more than 90% of metabolic products are phenolic conjugates and 50% of the conjugated metabolites were eugenol-glucuronide and eugenol-sulfate. Other observed metabolic routes were the epoxide-diol pathway, synthesis of a thiophenol and of a substituted propionic acid, allylic oxidation, and migration of the double bond [18]. In an in vitro study, all assessed metabolites have been found to have an aromatic hydroxyl group. The reaction of the hydroxyl group with glucoronate or sulfate causes the formation of conjugates which are finally excreted in the urine. Similarities have been observed between the metabolism of eugenol in human and rodents [19]. Guenette et al [20]. suggested that in rats, the half-life (t1/2) of eugenol in plasma is about 14 hours and in blood is 18 hours.
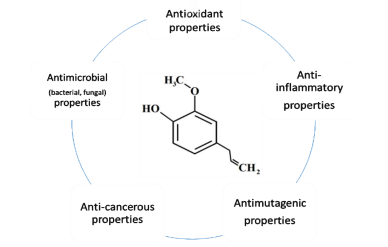
Figure 2. General applications of eugenol.
Source of Eugenol
Eugenol can be divided into biological extraction and chemical synthesis.
1. The natural eugenol mainly uses the volatile oil obtained by distillation from the dried flower buds of myrtle plant cloves, which is rich in clove oil. After extraction (a certain amount of sodium hydroxide solution dissolved separation of phenol and non-phenolic oil, and then by petroleum ether extraction, acidification, neutralization, distillation and other steps, Figure 3.
2. Synthesis method to prepare eugenol, using guaiacol as raw material, using allyl chloride, allyl alcohol and other direct allylation, Figure 4.
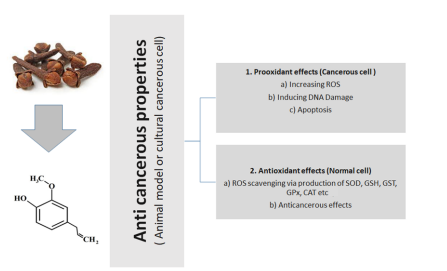
Figure 3. General anticancerous action of eugenol (tried and tested in animal or cultural cancerous cell models

Figure 4. Synthesis of eugenol
References
[1] Barceloux DG. Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants and Venomous Animals, Wiley: Hoboken; New Jersey; 2008.
[2] Basch E, Gasparyan A, Giese N, Hashmi S, Miranda M, Sollars D, Seamon E, Tanguay-Colucci S, Ulbricht C, Varghese M, Vora M, Weissner W. Clove (Eugenia aromatica) and clove oil (eugenol). Natural standard monograph (www.naturalstandard.com) copyright© 2008. J Diet Suppl. 2008;5:117- 146.
[3] Barnes J, Anderson L, Phillipson D. Herbal Medicine. Pharmaceutical Press; London; 2007.
[4] Oyedemi SO, Okoh AI, Mabinya LV, Pirochenva G, Afolayan AJ. The Proposed mechanism of bactericidal action of eugenol, α-terpineol and terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. Afr J Biotechnol. 2009;8:1280-1286.
[5] Kamatou GP, Vermaak I, Viljoen AM. Eugenol; from the remote Maluku Islands to the international market place: a review of a remarkable and versatile molecule. Molecules. 2012;17:6953-6981.
[6] Chapter 6-Clove Oil (Eugenol), https://www.marinwater.org/Document Center/View/253, Acccessed: 20 September 2016.
[7] Thompson DC, Constantin-Teodosiu D, Moldéus P. Metabolism and cytotoxicity of eugenol in isolated rat hepatocytes. Chem Biol Interact. 1991;77:137-147.
[8] Tammannavar, P.; Pushpalatha, C.; Jain, S.; Sowmya, S.V. An unexpected positive hypersensitive reaction to eugenol. Case Rep. 2013, 2013, bcr2013009464.
[9] Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689.
[10] Islam, S.S.; Al-Sharif, I.; Sultan, A.; Al-Mazrou, A.; Remmal, A.; Aboussekhra, A. Eugenol potentiates cisplatin anti-cancer activity through inhibition of ALDH-positive breast cancer stem cells and the NF-κB signaling pathway. Mol. Carcinog. 2018, 57, 333–346.
[11] Nejad, S.M.; Özgüne¸s, H.; Ba¸saran, N. Pharmacological and Toxicological Properties of Eugenol. Turk. J. Pharm. Sci. 2017, 14, 201–206.
[12] Pavithra, B. Eugenol—A Review. J. Pharm. Sci. Res. 2014, 6, 153–154.
[13] Pytko-Polo ´nczyk, J.; Muszy ´nska, B. Surowce naturalne w stomatologii. Med. Int. Rev. 2016, 27, 68–75.
[14] Navarro Triviño, F.J.; Barrales, C.; Ruiz-Villaverde, R. Eugenol allergy mimicking aphtous oral recurrent and burning mouth syndrome. Contact Dermat. 2019, 81, 462–463.
[15] abuto, H.; Tada, M.; Kohno, M. Eugenol [2-Methoxy-4-(2-propenyl) phenol] Prevents 6-HydroxydopamineInduced Dopamine Depression and Lipid Peroxidation Inductivity in Mouse Striatum. Biol. Pharm. Bull. 2007, 30, 423–427.
[16] Gülçin, I. Antioxidant activity of eugenol: A structure–activity relationship study. J. Med. Food 2011, 14, 975–985.
[17] Kim, D.Y.; Won, K.J.; Hwan, D.I.; Park, S.M.; Kim, B.; Lee, H.M. Chemical composition, antioxidant and anti-melanogenic activities of essential oils from Chrysanthemum boreale Makino at different harvesting stages. Chem. Biodivers. 2018, 15, e1700506.
[18] Fisher IU, von Unruh GE, Dangler HJ. The metabolism of eugenol in man. Xenobiotica. 1990;20:209-222.
[19] Eugenol and Related Hydroxyallylbenzene Derivatives, http://www. inchem.org/documents/
jecfa/jecmono/v56je09.pdf, Accessed: 10 August 2016.
[20] Guenette SA, Ross A, Beaudry F, Vachon P. Pharmacokinetics of eugenol and its effects on thermal hypersensitivity in rats. Eur J Pharmacol. 2007;562:60-67.
-
General description
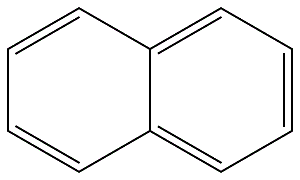
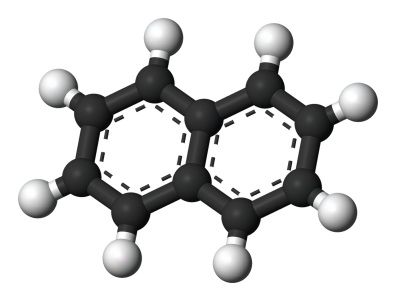
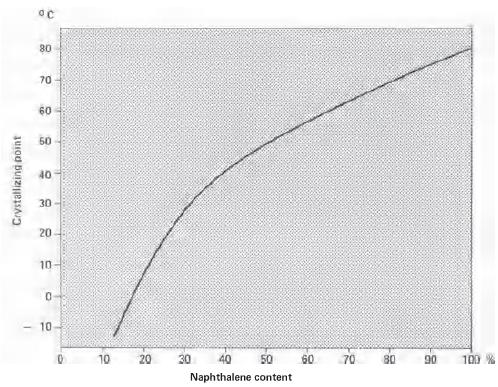
Naphthalene (CAS No: 91-20-3), the simplest bicyclic aromatic ring, is conventionally derived from coal tar. It is a white solid material with strong pungent odour. Naphthalene was first discovered by Scottish chemist, Alexzander garden in 1819. Michael Faraday first reported its molecular formula (C10H8) in 1826 [1]. Conventionally, naphthalene is synthesized by Diels-Alder reaction of maleic anhydride with 1,1 diaryl ethylene, followed by aromatisation of bis product by decarboxylation with barium hydroxide and copper. Further, phenyl substituted naphthalene is produced by Wagner-Jauregg reaction [2]. Organic compounds like naphthalene i.e. pconjugated ring system, have been the main priority in both academia and industry research for the past few decades. The naphthalene derived bioactive phytoconstituents include anticancer agents podophyllotoxins (Etoposide, teniposide) [3], and bis-ANS 82, an inhibitor of tubulin polymerisation and also an alkylating agent [4]. Also, Rifampicin is used as an antitubercular agent [5], whereas, justiprocumin A, B and patentiflorin A act as anti-HIV agents [6]. Naphthalene is cytotoxic and can be used for several therapeutic activities. Naphthalene epoxides and naphthoquinones are the reactive metabolites of naphthalene, responsible for the covalent interaction with cysteine amino acid of cellular proteins and cytotoxicity. Sulfhydryl groups of cysteine residues can react with both naphthalene oxides (epoxide) by SN2 and SN1 reactions and quinone metabolites of naphthalene (1,4- and 1,2-naphthoquinone) produced by 1,4-Michael addition reaction [7].

Figure 1 the molecular formula of naphthalene
Synthetic pathways of naphthalene
The syntheses of substituted naphthalenes have been reported across the literature of organic chemistry, but the development of new and useful regioselective synthesis methodologies of polysubstituted naphthalene derivatives have attracted much attention of the pharmaceutical industries [8]. Xiaoxia Zhang et al. synthesized naphthalenes and 2-naphthols by the electrophilic cyclization of alkynes. Different polysubstituted naphthalenes were prepared regioselectively under mild reaction conditions by 6-endo-dig electrophilic cyclization of suitable arene-containing propargylic alcohols by I2, Br2, NBS, and PhSeBr. The cyclization of analogous of 1-aryl-3-alkyn-2-ones resulted 3-iodo-2-naphthols in excellent yields [9]. Fukutani et al. synthesized highly substituted naphthalene derivatives by rhodium-catalysed oxidative coupling of aryl boronic acids with two alkynes molecules. They also performed homologation of benzene to naphthalene and naphthalene to anthracene by coupling of aromatic substrate with alkyne molecules in DMF or DMSO at 100C [10]. Chao Feng et al. synthesized highly substituted naphthalene derivatives by palladium-catalysed bisolefination of C-C triple bonds. The reactions were carried out at 110 C using substituted 1-ethynyl-2- vinylbenzene (0.1 mmol), substituted prop-1-enes (0.3 mmol) and PdCl2 (0.005 mmol) as a catalyst, in DMSO (0.4 mL) for 24 h under an oxygen atmosphere [11]. This nickel-catalysed cocyclotrimerization reaction of arynes with diynes was successfully producedsubstituted naphthalene derivatives in moderate to good yields.
Pharmacological implications
Naphyrone (O-2482), derived from pyrovalerone, is a psychoactive drug acting as norepinephrine-dopamine reuptake inhibitor (NDRI) with stimulant effect [12]. Tolnaftate (INN) is a synthetic anti-fungal thiocarbamate derivative, drug that inhibits squalene epoxidase, a vital enzyme in the ergosterol biosynthesis [13]. Naftifine, an allyl amine-containing a drug, is used as topical antifungal agent [14]. It also has anti-bacterial and anti-inflammatory actions also selectively blocks sterol biosynthesis via inhibition of the squalene 2,3-epoxidase enzyme [15]. Nafcillin is a narrow spectrum beta-lactam antibiotic of penicillin class, and issued to treat infections caused by gram-positive bacteria [16]. Terbinafine is another antifungal drug, which blocks ergosterol biosynthesis by inhibiting squalene epoxidase and thus catalyses the conversion of squalene to lanosterol [17]. Propranolol is a naphthalin containing drug and is used as b-adrenergic blocking agent to treat high blood pressure and irregular heart rate [18]. Duloxetine, a naphthalene containing heterocyclic antidepressant, acts as selective neurotransmitter reuptake inhibitor (SNRI) for serotonin, norepinephrine, and to a lesser degree dopamine [19]. Duloxetine is used inmajor depressive disorders [20] generalised anxiety disorders [21], The various activities associated with naphthalene derivatives, include anticancer, anti-microbial, anti-inflammatory, antiviral, antitubercular, antihypertensive, antidiabetic, antineurodegenerative, anti-psychotic, anti-convulsant and antidepressant activities.
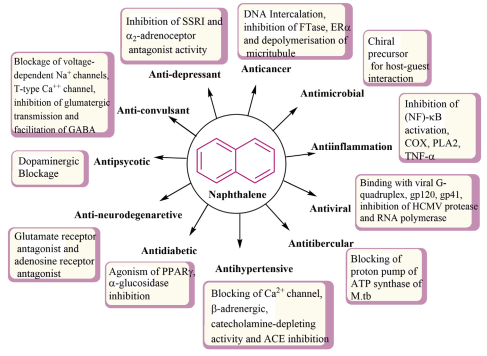
Figure 2 Various pharmacological effects of naphthalene derivatives
References
[1] J.M. Thomas, The genius of michael faraday, Eng. Sci. 55 (1992) 21e27.
[2] F. Bergmann, J. Szmuszkowicz, G. Fawaz, The condensation of 1, 1-diarylethylenes with maleic anhydride, J. Am. Chem. Soc. 69 (1947)1773e1777.
[3] K. Vogel, J. Sterling, Y. Herzig, A. Nudelman, a-1-Tributyltin-O-2, 3-bisacetyl-4, 6-ethylidene-glucose as a convenient glycosidation reagent: an efficientsynthesis of etoposide, Tetrahedron 52 (1996) 3049e3056.
[4] R.F. Luduena, M.C. Roach, P. Horowitz, The effects of the anilinonaphthalenesulfonates on the alkylation of tubulin: correlation between the appearance of sulfhydryl groups and apolar binding sites, Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 873 (1986) 143e146.
[5] M. Asif, Rifampin and their analogs: a development of antitubercular drugs, World J. Org. Chem. 1 (2013) 14e19.
[6] H. Zhang, D. Soejarto, L. Rong, H.H. Fong, E. Rumschlag-Booms, Aryl naphthalide lignans as anti-hiv agents, in: Google Patents, 2014.
[7] J. Zheng, M. Cho, A.D. Jones, B.D. Hammock, Evidence of quinone metabolites of naphthalene covalently bound to sulfur nucleophiles of proteins of murine Clara cells after exposure to naphthalene, Chem. Res. Toxicol. 10 (1997) 1008e1014.
[8] D.G. Batt, G.D. Maynard, J.J. Petraitis, J.E. Shaw, W. Galbraith, R.R. Harris, 2- Substituted-1-naphthols as potent 5-lipoxygenase inhibitors with topical antiinflammatory activity, J. Med. Chem. 33 (1990) 360e370.
[9] X. Zhang, S. Sarkar, R.C. Larock, Synthesis of naphthalenes and 2-naphthols by the electrophilic cyclization of alkynes, J. Org. Chem. 71 (2006) 236e243.
[10] T. Fukutani, K. Hirano, T. Satoh, M. Miura, Synthesis of highly substituted naphthalene and anthracene derivatives by rhodium-catalyzed oxidative coupling of arylboronic acids with alkynes, Org. Lett. 11 (2009) 5198e5201.
[11] C. Feng, T.-P. Loh, Palladium-Catalyzed Bisolefination of C C Triple Bonds: a facile method for the synthesis of naphthalene derivatives, J. Am. Chem. Soc. 132 (2010) 17710e17712.
Related articles And Qustion
See also
Lastest Price from Naphthalene manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 91-20-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500mt/year

US $9.00/KG2024-05-29
- CAS:
- 91-20-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000tons