Synthesis and Pharmaceutical Applications of 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one
The compound 2-[(1S,2S)-1-ethyl-2-benzyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one is a white to off-white powder, exhibiting notable fluorescent properties and demonstrating excellent chemical stability, which primarily serves as a pharmaceutical intermediate with documented research applications indicating its utility as a key synthetic intermediate in the preparation of the pharmacologically active molecule posaconazole.
Synthesis
Method 1
![Synthesis of 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one Article illustration](/NewsImg/2025-11-06/6389805982025676304614744.jpg)
Figure 1: Synthesis of 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one
In a nitrogen atmosphere, the reactant (110 mmol, 20.6 g) is charged into a 1 L reaction flask and dissolved in methyl tert-butyl ether (110 mL) at room temperature, followed by the slow addition of N,O-bis(trimethylsilyl)acetamide (53.8 mL, 220 mmol). After stirring the mixture for 1.5 hours at room temperature, ethyl magnesium bromide (146.8 mL, 440 mmol) is added dropwise at -30°C, and the reaction is allowed to proceed overnight. The mixture is quenched at -30°C with saturated ammonium chloride solution (50 mL), stirred for 30 minutes, and the organic layer is separated. The aqueous phase is extracted with ethyl acetate, and the combined organic phases are washed with saturated sodium chloride solution (50 mL) and water (50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting crude product is then combined with phenyl N-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]carbamate (38.9 g, 100 mmol) and triethylamine (25.2 mL, 200 mmol) in a 250 mL reaction flask. After adding 1,4-dioxane (100 mL), the mixture is reacted at 110°C under nitrogen for 24 hours. Water (20 mL) is added to precipitate a large amount of solid, which is isolated by suction filtration. The filter cake is collected, and the residue is recrystallized from toluene to obtain the final product 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one. [1]
Method 2
![Synthesis of 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one Article illustration](/NewsImg/2025-11-06/6389805986829566828122352.jpg)
Figure 2: Synthesis of 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one
A mixture of N'-((2S,3 S)-2,(benzyloxy)pentan-3-yl)formohydrazide compound of formula-17 (45.5 g) obtained in example-13, dioxane (500 ml) added phenyl 4-(4-(4-hydroxyphenyl)piperazin-1-yl)phenylcarbamate compound of formula-19 (50 g) was heated to 90-100°C. Triethylamine (26 g) was added to the reaction mixture at 90-100°C over a period of 1 hour and stirred for 24 hours at 90-100°C. After completion of the reaction, the reaction mixture was cooled to 25-30°C and dichloromethane was added to the reaction mixture. Filtered the reaction mixture through hyflow bed and washed with dichloromethane. Water was added to the filtrate. Both organic and aqueous layers were separated and the aqueous layer was extracted with dichloromethane. Both organic layers were combined and washed with 2% sodium hydroxide solution followed by water, and then with 5% hydrochloric acid solution followed by water and 5% NaHCO3 solution washing. Distilled off the solvent from organic layer under reduced pressure to get the title compound. Isopropyl alcohol (75 ml) was added to the obtained compound and the reaction mixture was cooled to 25-30°C. The reaction mixture was stirred for 6 hours at 25-30°C. Filtered, washed with isopropyl alcohol and then dried to get the title compound 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one. [2]
Pharmaceutical Applications
2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one serves as a pivotal synthetic intermediate in the pharmaceutical manufacturing sector, primarily employed in the sophisticated preparation of the antifungal agent posaconazole, which is a structurally optimized derivative of itraconazole that received FDA approval in 2006 as a second-generation triazole antifungal drug. As a therapeutically enhanced molecule derived from itraconazole, posaconazole has progressed to Phase III clinical trials, demonstrating a pharmacological mechanism consistent with azole-class agents while exhibiting superior potency in inhibiting sterol C14 demethylation—particularly against Aspergillus species—compared to its predecessor. The clinical applicability of posaconazole encompasses the treatment of fungemia caused by Candida and Cryptococcus species, along with respiratory, gastrointestinal, and urinary tract mycoses, as well as severe systemic infections including peritonitis and meningitis, thereby establishing its broad-spectrum antifungal profile.
Reference
[1] Xiao, Xiao; et al, Nitrite-Catalyzed Stereoselective Bromocyclization to Access Tetrahydrofuran Scaffolds under Batch and Flow: An Economic and Sustainable Approach to Gram-Scale Total Synthesis of Posaconazole, Organic Letters 2025, 27, 8281-8287.
[2] Reddy, Manne Satuamarauama; et al, Process for preparation of the triazole antifungal drug posaconazole, its intermediates and polymorphs, India Patent, Patent Number:WO2013042138.
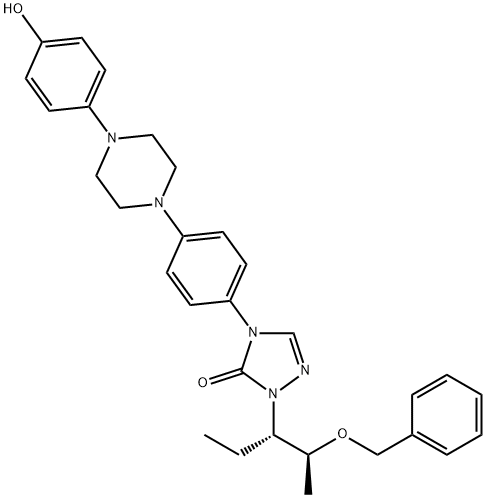 2-[(1S,2S)-1-ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]- 3H-1,2,4-Triazol-3-one
2-[(1S,2S)-1-ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]- 3H-1,2,4-Triazol-3-one 184177-83-1
You may like
See also
Lastest Price from 2-[(1S,2S)-1-ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]- 3H-1,2,4-Triazol-3-one manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 184177-83-1
- Min. Order:
- 1KG
- Purity:
- 98%-101%
- Supply Ability:
- 100 KG

US $0.00-0.00/mg2025-03-04
- CAS:
- 184177-83-1
- Min. Order:
- 10mg
- Purity:
- 98%
- Supply Ability:
- 500mg