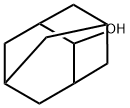
Properties and Applications of the 2-Adamantanol
General Introduction
2-Adamantanol is a white crystalline powder compound, soluble in organic solvents such as DMSO. 2-Adamantanol and its derivatives have specific biological activity and are used for the production of drugs with a wide range of antiviral activity. 2-Adamantanol is primarily used for the synthesis of the antiallergic drug mizolastine (Mizollen), and is also applied in the preparation of fragrances. It can also serve as a recrystallization solvent, a thermostat filler, and an organic synthesis intermediate. It should be stored in a sealed, dark, cool, and dry place, away from oxidizers. 2-Adamantanol poses a slight hazard to the aquatic environment and requires controlled discharge.[1]

Figure 1: The Picture of 2-Adamantanol
Bromination Reaction
The hydroxyl unit in the 2-adamantanol structure exhibits diverse chemical reactivity, being capable of undergoing oxidation in the presence of suitable oxidizing agents, as well as bromination when treated with halogenating reagents such as NBS. Experimental procedure for bromination reaction:A mixture of 2-adamantanol (0.9–1.1 mmol, 1 equiv) and N,N′-dimethylthiourea (DMTU, 0.45 equiv) in dry dichloromethane (4 mL) was stirred at room temperature until complete dissolution of the starting materials, followed by vigorous stirring and single-portion addition of N-bromosuccinimide (1.5 equiv). After reaction completion, the mixture was diluted with additional DCM (5 mL), and an aliquot (1 μL) was analyzed by GC–MS. The organic layer was then concentrated under reduced pressure, and the resulting crude product was purified by flash chromatography on silica gel using n-hexane (100%) as the eluent to afford the corresponding bromides. [2]
Oxidation Reaction
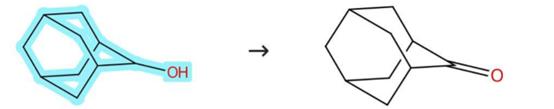
Figure 2: The Oxidation route of 2-Adamantanol
Perform a series of catalytic oxidation reaction in acetonitrile solution at room temperature, under open air. Set the reaction by using 1 mmol of 2-Adamantanol, 2.5 mol % of copper(I)iodide, 6 mol % of 2, 2'-dipyridylamine and 6 mol % of 9-azabicyclo[3.3.1]nonane N-oxyl in 3 mL acetonitrile and heat not apply. Set the oxidation reaction in a 20 ml test tube and equip with a magnetic stirrer bar. Stir the reaction at 1500 rpm for 12 h. [3]
Substitution Reaction
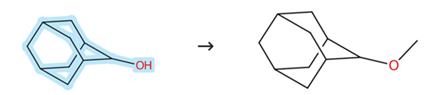
Figure 3: Substitution route of 2-Adamantanol
Add the 2-Adamantanol (5 mmol, 1.0 equiv) to a stirring suspension of NaH (7.5 mmol, 1.5 equiv.) in THF (0.2 M) at 0°C. Add MeI (12.5 mmol, 2.5 equiv.) in one portion at 0°C after 5 min. Warm the reaction to room temperature and stir for 5-15 h. Cool the mixture to 0°C and quench by the dropwise addition of a saturated aqueous solution of NH4Cl (caution: H2 evolution). Extract the aqueous layer with ethyl acetate (3 × 20 mL). Dry the combined organic layers with Na2SO4, filter and concentrate under reduced pressure. Purify the crude mixture by flash chromatography (hexane/EtOAc = 20/1).[4]
Hydrogen Bond Interaction
In low-temperature crystal structures, 2-adamantanol exhibits asymmetric units comprising three molecules that form hexameric clusters through hydrogen bonding. No high-resolution gas-phase spectroscopic studies have been reported for this compound. Stark modulation microwave spectroscopy of 1-adamantanol determined an internal rotation barrier of 4.9(4) kJ·mol⁻¹ for the alcohol group, consistent with values observed for other alcohols. The jet-cooled FT-IR spectrum of 2-adamantanol recorded by Suhm revealed OH stretching bands at 3650 cm⁻¹ for the monomer and 3520 cm⁻¹ for the dimer; however, the single red-shifted dimer band precluded confirmation of potential isomers. Solid-state FT-IR spectral data are available for the 1-adamantanol monomer. The monohydrate and dimer of 2-adamantanol serve as model clusters that integrate a relatively strong electrostatically dominated O-H··O hydrogen bond with a moderately sized, highly symmetric aliphatic sidechain consisting of ten carbon atoms. These dimeric systems enable detailed investigation of multiple aspects of non-covalent interactions, such as the competition between the primary hydroxyl hydrogen bond and interactions involving the aliphatic group, the balance of intramolecular and intermolecular forces, conformational equilibria under jet-cooled conditions, the presence of internal large-amplitude motions, and the performance of DFT computational methods. This work extends previous rotational spectroscopy studies on cyclohexanol monohydrate and dimer, thereby establishing a foundation for comparative analysis with analogous alcohol molecular systems. Both cyclohexanol and 2-adamantanol monohydrates similarly form a single isomer in which the water molecule functions as a proton donor. [5]
Reference
[1] M.B. Charapennikau, The heat capacities and parameters of solid phase transitions and fusion for 1- and 2-Adamantanols, J. Chem. Thermodynamics, 2003, 35, 145–157
[2] Amar R. Mohite, Thiourea-Mediated Halogenation of Alcohols, J. Org. Chem. 2020, 85, 20, 12901–12911.
[3] Heshmatnia Mild Aerobic Oxidation of Secondary Alcohols with Water Tolerant Cu(I) Catalyst,. ChemSusChem, 2025, 18, e202402236
[4] Zhao, Yuhui; Nanoporous Gold Catalyzed Borylation of C(sp3)-O Bonds in Dialkyl Ethers and Mechanistic Elucidation. ACS Catalysis, 2025, 15, 13118-13126
[5] Juanes M, Saragi R T, Pérez C, et al. Hydrogen Bonding in the Dimer and Monohydrate of 2-Adamantanol: A Test Case for Dispersion-Corrected Density Functional Methods[J]. Molecules, 2022, 27 : 2584.
Lastest Price from 2-Adamantanol manufacturers
US $1.10/g2025-09-17
- CAS:
- 700-57-2
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min

US $5.00-0.50/KG2025-05-30
- CAS:
- 700-57-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS