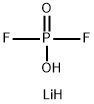Lithium Difluorophosphate (LiDFP) Additive: Functions, Mechanisms, and Applications in Lithium-Based Batteries
LiDFP as aDendrite-Suppressing Electrolyte Additive
The growth of lithium dendrite and the low Coulombic efficiency (CE) of Li anode during repeated Li plating/stripping processes are the two major obstacles to the development of rechargeable Li metal batteries (LMBS). Herein, Lithium difluorophosphate (LiDFP) is used as an electrolyte additive to suppress the growth of Li dendrites by forming a vigorous and stable solid electrolyte interphase layer on the Li metal surface. Moreover, the Li CE can be largely improved from 84.6% for the conventional LiPF6-based electrolyte to 95.2% by the addition of an optimal concentration of LiDFP at 0.15 M. The optimal LiDFP-containing electrolyte can allow the Li||Li symmetric cells to cycle stably for more than 500 and 200 h at 0.5 and 1.0 mA cm-2, respectively, much longer than the control electrolyte without LiDFP additive. Meanwhile, this LiDFP-containing electrolyte can also greatly enhance the cycle life of the Li||LiNi1/3Co1/3Mn1/3O2 cells with a moderately high mass loading of 9.7 mg cm-2. These results demonstrate that LiDFP has extensive application prospects as a dendrite-suppressing additive in advanced LMBs. [1]

Widely Applicable SEI/CEI-Forming Mechanism of LiDFP
Lithium difluorophosphate (LiDFP) is among the most widely applicable additives used to construct a robust solid electrolyte interphase (SEI) on electrode, which can improve the cycling performance and rate performance of high-voltage cathodes and Li metal anodes at extreme temperatures. The most reported mechanism for the function of LiDFP involves the formation of robust SEI/CEI on both cathode and anode. This mechanism is deduced from the initial redox propensity and the morphology and component analysis results of the derived SEI/CEI. In addition, LiDFP can increase the solvent stability by increasing the LUMO energy of the carbonate solvent-DFP– anion cluster through the electrostatic inductive effect in the solvent-anion cluster.[12] This property is common for almost all salt anions. The element analysis of X-ray photoelectron spectroscopy (XPS) and time of flight secondary ion mass spectrometry (TOF-SIMS) results show that the SEI/CEI formed in the LiDFP-containing electrolytes is rich in P and F containing inorganics, such as Li3PO4 and LiF. Moreover, the P=O rich inorganic interphase is generally an excellent Li+ ion conductor, endowing the battery with low interfacial impedance. [2]
LiDFP in High-Voltage LIBs: Performance Benefits and Thermal Stability Issues
The specific energy/energy density of state-of-the-art (SOTA) Li-ion batteries can be increased by raising the upper charge voltage. However, instability of SOTA cathodes (i. e., LiNiyCoxMnyO2; x+y+z=1; NCM) triggers electrode crosstalk through enhanced transition metal (TM) dissolution and contributes to severe capacity fade; in the worst case, to a sudden death (“roll-over failure”). Lithium difluorophosphate (LiDFP) as electrolyte additive is able to boost high voltage performance by scavenging dissolved TMs. However, LiDFP is chemically unstable and rapidly decomposes to toxic (oligo)organofluorophosphates (OFPs) at elevated temperatures; It is shown, that fluoroethylene carbonate (FEC) suppresses OFP formation, though being unsuitable for high voltage application as single additive. Nevertheless, combined with LiDFP, the advantage of each individual additive gets pronounced, that is, high voltage performance in NCM||graphite cells at 4.5 V (LiDFP) and OFP suppression (FEC). [3]
LiDFP-Derived Cathode Coatings for Enhanced All-Solid-State Battery Performance
In this study, the additives lithium difluorophosphate (LiDFP) and lithium difluoro(oxalato)borate (LiDFOB) are used to fabricate stable cathode coatings via heat treatment. The low melting points of LiDFP and LiDFOB enable the formation of thin and uniform coating layers by a low-temperature heat treatment. All-solid-state cells containing LiDFP- and LiDFOB-coated cathodes show electrochemical performances significantly better than those comprising uncoated cathodes. Among all of the as-prepared coated cathodes, LiDFP-coated cathodes fabricated using a slightly lower temperature than the phase-transition temperature of LiDFP (320 °C) show the best discharge capacity, rate capability, and cyclic performance. Furthermore, cells comprising LiDFP-coated cathodes showed significantly low impedance. LiDFP-coated cathodes minimized side-reactions during cycling, resulting in a significantly low cathode-surface degradation. [4]References:
[1] Shi, P., Zhang, L., Xiang, H., Liang, X., Sun, Y., & Xu, W. (2018). Lithium difluorophosphate as a dendrite-suppressing additive for lithium metal batteries. ACS applied materials & interfaces, 10(26), 22201-22209.
[2] Wang, A., Wang, L., Liang, H., Song, Y., He, Y., Wu, Y., ... & He, X. (2023). Lithium difluorophosphate as a widely applicable additive to boost lithium‐ion batteries: a perspective. Advanced Functional Materials, 33(8), 2211958.
[3] Kubot, M., Frankenstein, L., Muschiol, E., Klein, S., Esselen, M., Winter, M., ... & Kasnatscheew, J. (2023). Lithium Difluorophosphate: A Boon for High Voltage Li Ion Batteries and a Bane for High Thermal Stability/Low Toxicity: Towards Synergistic Dual Additives to Circumvent this Dilemma. ChemSusChem, 16(6), e202202189.
[4] Joo, M. J., Kim, M., Chae, S., Ko, M., & Park, Y. J. (2023). Additive-Derived Surface Modification of Cathodes in All-Solid-State Batteries: The Effect of Lithium Difluorophosphate- and Lithium Difluoro(oxalato)borate-Derived Coating Layers. ACS applied materials & interfaces, 15(51), 59389–59402. https://doi.org/10.1021/acsami.3c12858
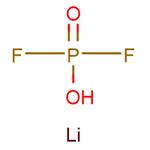
US $0.00/KG2025-08-22
- CAS:
- 24389-25-1
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 1ton
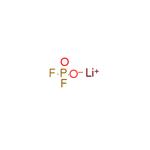
US $1.10-9.90/kg2025-08-18
- CAS:
- 24389-25-1
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 100kg