How Abemaciclib works in treating breast cancer
Description of Abemaciclib
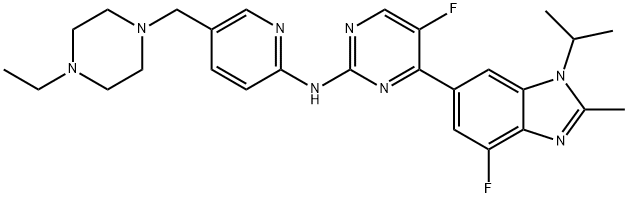
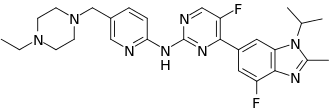
Abemaciclib(brand name:Verzenios) is an oral, targeted antineoplastic agent belonging to a class of reversible cell cycle protein-dependent kinase 4 (CDK4) and 6 (CDK6) inhibitors. Abemaciclib is primarily used for the treatment of HR+ HER2-advanced or metastatic breast cancer.

Treatment with Abemaciclib
Abemaciclib is currently FDA approved as initial endocrine-based treatment for postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer at a dose of 150mg twice daily in combination with an aromatase inhibitor. Abemaciclib is also FDA approved for HR-positive, HER2-negative advanced or metastatic breast cancer after disease progression with prior endocrine therapy at 150mg twice daily in combination with fulvestrant. Additionally, abemaciclib monotherapy is FDA approved at 200mg twice daily for HR-positive, HER-2 negative advanced or metastatic breast cancer that has progressed after previous chemotherapy and endocrine therapy[1].
Mechanism of action
In normal cellular physiology, CDK 4 and CDK 6 promote passage from the G1 phase to the S phase of the cell cycle and are fundamental for the regulation of cell proliferation. Inhibitors of cyclin-dependent kinase cyclin (CDK4/6i) currently available in the clinic fall into the class of cytostatic agents. They interact with the ATP-binding pocket of CDK4 and 6 and display a higher selectivity for CDK4 and CDK6 as compared with CDK1 and CDK2. Unlike other CDK inhibitors (e.g. Palbociclib and Ribociclib), abemaciclib exhibits greater selectivity for CDK4 compared to CDK6.
Preclinical studies have shown that continuous inhibition of CDK4/6 by abemaciclib inhibits phosphorylation of the retinoblastoma protein, which becomes inactivated. This effect, in turn, blocks the transition of neoplastic ACCEPTED MANUSCRIPT Information Classification: General cells from the G1 to the S phase and the release of the transcription elongation factor 2 required for cell-cycle progression resulting in apoptosis or senescence[2].
Side effects
Abemaciclib Very common side effect and can sometimes be serious. Diarrhoea is most likely to occur during the first week of treatment. It may last for about two weeks. If you have loose stools, you are advised to start taking an anti-diarrhoeal medicine immediately, drink plenty of fluids and contact your doctor.
In addition, abemaciclib may also be associated with a low white blood cell count, anaemia, a decrease in the number of platelets, yellowing of the skin or whites of the eyes (jaundice), pain in the right side of the lower ribs, bleeding or bruising more easily than usual, fatigue, nausea, vomiting, and rash.
References:
[1] WRIGHTMATTHEW D; AbrahamMd J. Preclinical discovery and development of abemaciclib used to treat breast cancer.[J]. Expert Opinion on Drug Discovery, 2021, 16 5: 485-496. DOI:10.1080/17460441.2021.1853097.
[2] GEBBIAVITTORIO. Abemaciclib: safety and effectiveness of a unique cyclin-dependent kinase inhibitor.[J]. Expert Opinion on Drug Safety, 2020, 19 8: 945-954. DOI:10.1080/14740338.2020.1781814.
You may like
Related articles And Qustion
Lastest Price from Abemaciclib manufacturers

US $1.10-9.90/kg2025-08-15
- CAS:
- 1231929-97-7
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 100kg

US $5.00-0.50/KG2025-06-05
- CAS:
- 1231929-97-7
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS