N,N,N',N'-Tetramethyldiaminomethane: properties, applications and safety
General Description
N,N,N',N'-Tetramethyldiaminomethane is a colorless liquid with a pungent odor and versatile properties that make it an indispensable compound in organic synthesis and industrial processes. It is highly soluble in many polar solvents, has a relatively high boiling point and low freezing point, and chelating ability with alkali metals such as lithium. N,N,N',N'-Tetramethyldiaminomethane finds various applications as a complexing agent in coordination chemistry, a solvent for high-temperature reactions, and has unique lithium ion affinities influenced by its molecular conformation. However, N,N,N',N'-Tetramethyldiaminomethane is a highly flammable and toxic substance that requires proper handling and safety measures to prevent accidents and harm. In summary, N,N,N',N'-Tetramethyldiaminomethane's versatile properties make it a valuable compound in industry, but its hazardous nature demands caution and adherence to safety protocols.

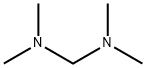
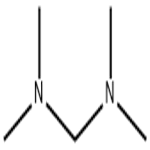
Figure 1. N,N,N',N'-Tetramethyldiaminomethane
Properties
N,N,N',N'-Tetramethyldiaminomethane is a colorless liquid with a pungent odor and a chemical formula of C5H14N2. Firstly, N,N,N',N'-Tetramethyldiaminomethane is highly soluble in many polar solvents such as water, alcohols and acetone due to its strong hydrogen bonding ability. This property makes it an ideal candidate for use as a complexing agent in coordination chemistry, particularly in metal-catalyzed organic reactions. Secondly, N,N,N',N'-Tetramethyldiaminomethane has a relatively high boiling point of 85°C, which allows it to be used as a solvent for high-temperature reactions and as a reaction promoter in the synthesis of polymers and pharmaceuticals. Additionally, this compound has a low freezing point of -55°C, making it useful for low-temperature reactions. Thirdly, N,N,N',N'-Tetramethyldiaminomethane is known for its ability to chelate with alkali metals such as lithium, sodium, and potassium, thereby forming stable complexes. This property is exploited in the preparation of Grignard reagents from alkyl and aryl halides, and in the synthesis of organometallic compounds. Overall, N,N,N',N'-Tetramethyldiaminomethane's solubility, boiling point, freezing point and chelating ability make it a versatile and indispensable compound in organic synthesis and industrial processes. 1
Applications
N,N,N',N'-Tetramethyldiaminomethane is a highly versatile compound with various applications. Its lithium ion affinities have been studied using density functional theory (DFT) and correlated ab initio methods. One important application of TMEDA is as a complexing agent in coordination chemistry. It can form stable complexes with alkali metals such as lithium, which is particularly useful in the synthesis of organometallic compounds and the preparation of Grignard reagents. The study mentioned that the conformational stability of TMEDA is influenced by anomeric effects, specifically n(N) → σ*(C-N) hyperconjugative interactions. This indicates that TMEDA's coordination abilities are affected by the electronic interactions between its nitrogen atoms and the lithium ion. The results suggest that TMEDA's lithium ion affinities are conformationally dependent, meaning that the arrangement of its molecular structure affects its interactions with lithium ions. Additionally, the size of the cyclical system can influence these affinities in 1,3-diaza monocyclic systems. In summary, N,N,N',N'-Tetramethyldiaminomethane finds applications as a complexing agent in coordination chemistry, a solvent for high-temperature reactions, and has unique lithium ion affinities influenced by its molecular conformation. 2
Safety
N,N,N',N'-Tetramethyldiaminomethane is a highly flammable liquid that poses significant safety risks. This compound can ignite easily if exposed to heat, sparks, or flames, making it crucial to handle with extreme caution. In addition, inhalation of TMEDA fumes can cause corrosive injuries to the upper respiratory tract and lungs. Moreover, TMEDA is known to cause severe skin burns and eye damage upon contact, including serious eye damage. Therefore, it is highly recommended to wear personal protective equipment, such as gloves and safety goggles, when handling this compound. According to the Intraperitoneal LD50 (mouse) test, TMEDA has a median lethal dose of 220 mg/kg, indicating its high toxicity. It has also been identified as a hazardous substance by the Right to Know Hazardous Substance Fact Sheets (RTECS) due to its ability to cause burns. In summary, TMEDA is a highly flammable compound that can cause severe skin burns, eye damage, and respiratory injuries. It is important to adhere to proper safety protocols while handling this compound to prevent any accidents or harm. 3
Reference
1. PubChem. COMPOUND SUMMARY: N,N,N',N'-Tetramethylmethanediamine. National Library of Medicine, 2005, PubChem CID: 5829.
2. Kesharwani MK, Thiel W, Ganguly B. Probing the influence of anomeric effects on the lithium ion affinity in 1,3-diaza systems: a computational study. J Phys Chem A. 2010 Oct 7;114(39):10684-10693.
3. Chemical Datasheet: TETRAMETHYLMETHYLENEDIAMINE. CAMEO Chemicals, UN/NA Number: 1993.
Related articles And Qustion
See also
Lastest Price from N,N,N',N'-TETRAMETHYLDIAMINOMETHANE manufacturers
US $0.00/KG2022-01-15
- CAS:
- 51-80-9
- Min. Order:
- 1KG
- Purity:
- 97.1%
- Supply Ability:
- 100 tons