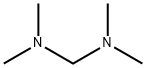
The role of N,N,N',N'-Tetramethyldiaminomethane in Mannich reaction
N,N,N',N'-Tetramethylmethylenediamine is a reagent used as a convenient source of the Mannich intermediate [1]. N,N,N',N'-Tetramethylmethylenediamine (1) can be used for the dimethylaminomethylation of active methylene compounds. Acidic conditions such as Trifluoroacetic Acid (TFA) (eq. a in scheme 1) or Phosphoric Acid in Acetic Acid (eq. b in scheme 1) catalyze the elimination of dimethylamine from, resulting in the formation of N,N‐dimethyl(methylene)ammonium salts. The in situ use of these salts in the Mannich reaction is usually more convenient than the direct use of the preformed N,N‐dimethyl(methylene)ammonium salts, since the latter are typically hygroscopic and difficult to handle [2].
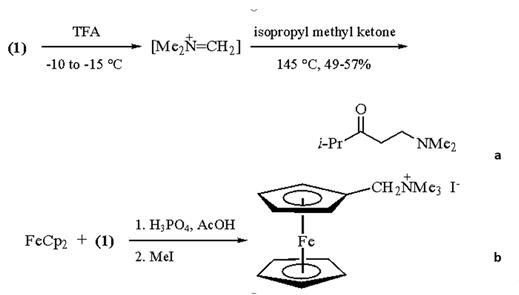
Scheme 1 the reaction of N,N,N',N'-Tetramethylmethylenediamine
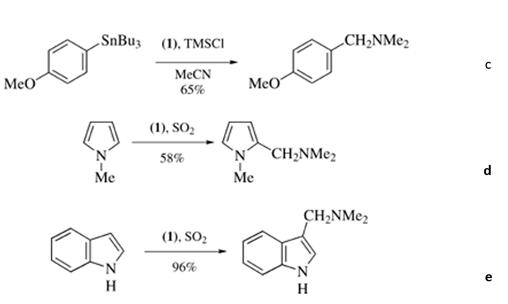
Scheme 2 The reaction of N,N,N',N'-Tetramethylmethylenediamine
Dimethylaminomethylations of aryltrialkylstannanes with N,N,N',N'-Tetramethylmethylenediamine (1) in the presence of silyl chlorides have been reported (eq. c in scheme 2). Reagent (1) also reacts with heterocyclic compounds such as N‐methylpyrroles (eq. d in scheme 2) and indoles (eq. e in scheme 2) in the presence of acetyl chloride or excess Sulfur Dioxide to provide the corresponding Mannich products.
As early as in 1973, Michel and his colleagues showed regioselective Mannich condensation with dimethyl(methylene)ammonium trifluoroacetate [3].
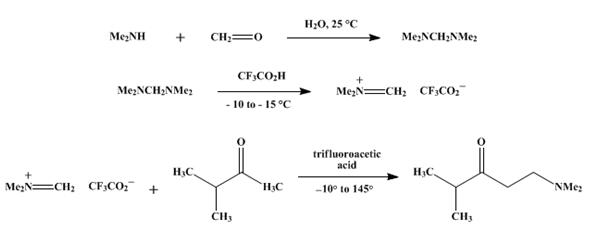
As shown in scheme 3. N,N,N',N'-Tetramethyldiaminomethane was added in a 500-ml., round-bottomed flask equipped with a magnetic stirring bar and a dropping funnel is charged with 100 g. (1.0 mol.) of aqueous 30% formaldehyde. The solution is stirred and cooled in an ice bath as 225 g. (2.0 moles) of a 40% solution of dimethylamine in water is added dropwise. The resulting aqueous solution is allowed to stand overnight at room temperature, after which it is saturated with solid potassium hydroxide. The two layers are separated, the upper layer is dried over potassium hydroxide pellets, and the drying agent is removed. Distillation at atmospheric pressure through a Vigreux column gives 85–88 g. (83–86%) of N,N,N',N'-Tetramethyldiaminomethane. The following reactions are similar as that in scheme 1.
The key point of the Mannich condensation has traditionally been carried out in the presence of water as a three-component condensation involving a carbonyl compound (or related carbon nucleophile), formaldehyde, and a primary or secondary amine. The initial step is a condensation between the latter two reactants to form a mono- or dialkyl(methylene)ammonium ion which subsequently serves as the electrophilic partner in the reaction. With unsymmetrical ketones aminomethylation generally occurs at both positions, giving mixtures of isomeric β-amino ketones. The ratio of the isomers depends strongly on the structure of the ketone, and the more highly branched β-amino ketone usually predominates. In the past decades, a number of methods have been developed for the preparation of dialkyl(methylene)ammonium salts (Mannich reagents), and their use in Mannich-type condensation reactions under anhydrous conditions has improved the scope and efficiency of this important synthetic process. However, the orientation of the Mannich reaction may nevertheless be difficult to control. Apart from the work of the submitters, the preparation of isomerically pure Mannich bases has only been achieved by indirect methods in which specific enol derivatives are generated and allowed to react with dialkyl(methylene)ammonium salts. The Mannich reaction of β-keto esters affords isomerically pure β-dimethylamino β'-keto esters which may in turn be converted to specific α-methylene ketones. However, the β-amino ketones themselves are not as yet available by this method.
Reference
[1] https://www.alfa.com/en/catalog/L04037/
[2] https://onlinelibrary.wiley.com/doi/full/10.1002/047084289X.rb143?rb143-eo-c00001
[3] Michel Gaudry, etc., Regioselective Mannich Condensation with Dimethyl(Methylene)Ammonium Trifluoroacetate: 1-(Dimethylamino)-4-Methyl-3-Pentanone, Organic Syntheses, 1979, 59, 153
You may like
Related articles And Qustion
Lastest Price from N,N,N',N'-TETRAMETHYLDIAMINOMETHANE manufacturers
US $0.00/KG2022-01-15
- CAS:
- 51-80-9
- Min. Order:
- 1KG
- Purity:
- 97.1%
- Supply Ability:
- 100 tons