N,N,N',N'-Tetramethyldiaminomethane: Overview and Applications in Organic Synthesis
General Description
N,N,N',N'-Tetramethyldiaminomethane is a versatile organic compound widely utilized in organic synthesis. Its strong basicity and stability make it valuable in various chemical reactions. Specifically, it is employed in the visible-light-induced synthesis of imidazolidines, crucial for developing new pharmacological drugs, showcasing its significance in modern organic synthesis. Additionally, TMEDA plays a crucial role in the preparation of 3-aryl-4H-chromene-4-ones, which are utilized as antineoplastic agents for the treatment of cancer. This application has shown significant inhibition of prostate cancer cells, highlighting the potential for novel cancer treatment strategies. Overall, N,N,N',N'-Tetramethyldiaminomethane's diverse applications underscore its importance in the development of pharmaceutical compounds and novel antineoplastic agents with promising therapeutic applications.

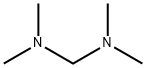
Figure 1. N,N,N',N'-Tetramethyldiaminomethane
Overview
N,N,N',N'-Tetramethyldiaminomethane, commonly known as tetramethylethylenediamine or TMEDA, is an organic compound with a distinct chemical structure. It features four methyl groups attached to a diamine backbone, conferring unique properties on the molecule. This amine exhibits strong basicity due to the presence of two amino groups, making it a valuable reagent in various chemical reactions. Additionally, its symmetric structure endows it with stability and predictability in reaction outcomes. In synthetic chemistry, N,N,N',N'-Tetramethyldiaminomethane is often employed as a ligand or a catalyst, promoting reactions such as polymerization and cross-coupling. Its solubility in organic solvents allows for facile manipulation in laboratory settings. However, it's crucial to handle N,N,N',N'-Tetramethyldiaminomethane with care due to its potential toxicity and flammability. Overall, N,N,N',N'-Tetramethyldiaminomethane is a versatile compound with numerous applications in the field of chemistry. 1
Applications in organic synthesis
Synthesis of imidazolidines
N,N,N',N'-Tetramethyldiaminomethane is a versatile compound with a wide range of applications, including its role in the visible-light-induced synthesis of imidazolidines. Imidazolidine, a saturated heterocycle with a cyclic aminal core, holds significant potential for the development of new pharmacological drugs. In the context of the mentioned study, it serves as a key component in the formal [3 + 2] cycloaddition reaction with aromatic imines. The process involves the use of heteroleptic cyclometalated Ir(III) complex photosensitizers, which play a crucial role in enabling the unprecedented synthesis of imidazolidines. Through a formal [3 + 2] photocycloaddition, aromatic imines are combined with N,N,N',N'-tetramethyldiaminomethane to produce these valuable compounds. The reaction mechanism, proposed based on quenching experiments, quantum yield, ground-state redox potentials, and excited-state potentials, provides insight into the intricate processes at play during the synthesis. This innovative application of N,N,N',N'-Tetramethyldiaminomethane showcases its significance in modern organic synthesis, particularly in the development of novel pharmaceutical compounds. As research in this area continues to progress, the potential for further advancements in drug discovery and development using imidazolidines and related compounds becomes increasingly promising. 2
Preparation of 3-aryl-4H-chromene-4-ones
N,N,N',N'-Tetramethyldiaminomethane plays a crucial role in the preparation of 3-aryl-4H-chromene-4-ones, which are utilized as antineoplastic agents for the treatment of cancer. In the disclosed process, isoflavonoids of a specific formula or their pharmaceutically acceptable salts are employed for treating prostate cancer or inhibiting prostate cancer metastasis. The compounds of formula I, where Ar represents an aryl or heteroaryl group and X can be halide, alkoxy, or form a cyclic ether structure, are key components in this application. The direct conversion of the corresponding Betti's base, facilitated by heating with acetic anhydride in the presence of potassium acetate, results in the formation of the desired 3-aryl-4H-chromene-4-one in high yield. Evaluation of candidate compounds for their antineoplastic activity against prostate cancer PC3 cells revealed significant inhibition, with one compound (II) exhibiting 17.1% ± 5.9% activity. By leveraging N,N,N',N'-Tetramethyldiaminomethane in the synthesis of these crucial compounds, researchers have paved the way for potential advancements in cancer treatment strategies, particularly in addressing prostate cancer and its metastasis. This underscores the importance of N,N,N',N'-Tetramethyldiaminomethane in the development of novel antineoplastic agents with promising therapeutic applications. 3
Reference
1. N,N,N',N'-Tetramethylmethanediamine. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 5829.
2. Kennosuke I. Visible-Light-Induced Synthesis of Imidazolidines Through Formal [3+2] Cycloaddition of Aromatic Imines with N,N,N’,N’-Tetramethyldiaminomethane. SSRN Electronic Journal. 2022; 22: 2.
3. Bondarenko SP, Frasinyuk MS, Liu C, Watt DS. Preparation of 3-aryl-4H-chromene-4-ones as antineoplastic agents for the treatment of cancer. 2016; Patent Number: US20160332981.
Related articles And Qustion
Lastest Price from N,N,N',N'-TETRAMETHYLDIAMINOMETHANE manufacturers
US $0.00/KG2022-01-15
- CAS:
- 51-80-9
- Min. Order:
- 1KG
- Purity:
- 97.1%
- Supply Ability:
- 100 tons