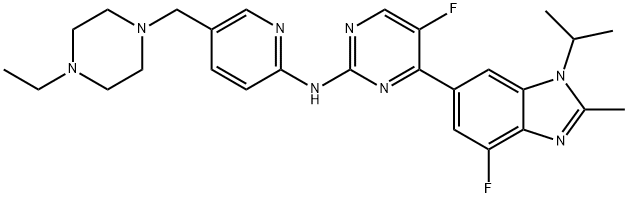
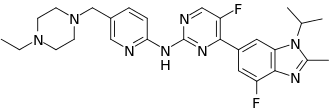
Abemaciclib: pharmacology, pharmacokinetics and mechanism of action
General Description
Abemaciclib is a prescription drug used to treat breast cancer. Abemaciclib is a potent inhibitor of CDK4 and CDK6, selectively targeting their complexes with cyclinD1. It acts by blocking the G1 to S phase transition of the cell cycle, inducing cell cycle arrest, apoptosis, and senescence in cancer cells. Compared to other CDK4/6 inhibitors, it has higher inhibitory activity on CDK4. Abemaciclib has good oral bioavailability, a mean half-life of 17.4-38.1 hours, and primarily undergoes hepatic metabolism via CYP3A4. It can be used as a single-agent or in combination therapy and has shown efficacy in inducing cell cycle arrest and metabolic alterations. Overall, abemaciclib's mechanism of action involves inhibiting cell cycle progression and promoting senescence and apoptosis in cancer cells.
Figure 1. Tablets of abemaciclib
Pharmacology
Abemaciclib is a potent inhibitor of CDK4 and CDK6, selectively targeting their complexes with cyclinD1. It exerts its action on these complexes even at nanomolar concentrations, with IC50 values of 2nmol/L for CDK4/cyclinD1 and 5nmol/L for CDK6/cyclinD1. Compared to other agents in the same class, Abemaciclib has higher inhibitory activity on CDK4 than CDK6. It can also cross the blood-brain barrier based on preclinical models. In normal cellular physiology, CDK4 and CDK6 play a crucial role in promoting cell cycle progression from the G1 phase to the S phase and regulate cell proliferation. Abemaciclib, as a synthetic compound, has been designed for oral administration and has a molecular weight of 506.606g/mol. While it may have some activity against CDK9, it primarily acts as a highly selective CDK4/6 inhibitor, being 12 times more potent against CDK4 and CDK6. It can be used as a single-agent or in combination therapy, independently of fasting conditions. 1
Pharmacokinetics
In phase I studies, the dose of abemaciclib was gradually increased up to 275 mg twice daily. The mean terminal elimination half-life of abemaciclib ranged from 17.4 to 38.1 hours, and there were no dose-dependent changes in clearance. At steady state, the mean maximum plasma concentrations were 249 ng/mL and 298 ng/mL for doses of 150 mg and 200 mg every 12 hours, respectively. The mean area under the plasma concentration-time curve over 24 hours reached 4280 and 5520 ng-h/mL for doses of 150 mg and 200 mg twice daily, respectively. After oral administration, the absolute bioavailability of abemaciclib is 45%, and the peak plasma concentration is typically observed after a median of 8 hours (range: 4.1-24.0 hours). Most of the drug and its metabolites (96.3%) bind to plasma proteins. The primary route of excretion is through the feces (81%), while renal filtration plays a lesser role. The median plasmatic half-life of abemaciclib is 18.3 hours. Abemaciclib is primarily metabolized in the liver by the enzyme CYP3A4, producing N-desethylabemaciclib and hydroxy derivatives (M18, M20), as well as another oxidative metabolite. It is important to note that potent CYP3A4 inhibitors such as ketoconazole and clarithromycin, as well as inducers like rifampicin, can significantly affect the exposure to abemaciclib and its active metabolites. Inhibitors of CYP3A4 can increase plasma levels of abemaciclib, leading to a risk of severe toxicity, while CYP3A4 inducers may cause lower plasma concentrations and potentially decrease the therapeutic effect. 2
Mechanism of action
Abemaciclib is a CDK4/6 inhibitor used in the treatment of cancer. It is administered orally and acts by continuously inhibiting CDK4/6, which are essential for cell proliferation regulation. By blocking the passage from the G1 to the S phase of the cell cycle, abemaciclib prevents neoplastic cells from progressing and induces cell cycle arrest, apoptosis, and senescence. In clinical studies, abemaciclib has shown a higher efficacy in inducing cell cycle arrest compared to other CDK4/6 inhibitors. Additionally, it causes extensive metabolic alterations in cancer cells, affecting mitochondrial function and reducing the concentration of specific metabolites involved in the TCA cycle. The transition from quiescence to senescence, known as geroconversion, leads to permanent growth arrest and the production of various molecules that make up the senescence-associated secretory phenotype. Overall, abemaciclib's mechanism of action involves cell cycle inhibition, metabolic changes, and induction of senescence and apoptosis in cancer cells. 3
Reference
1. Sanchez-Martinez C, Gelbert LM, Shannon H, et al Abstract B234: LY2835219, a potent oral inhibitor of the cyclin-dependent kinases 4 and 6 (CDK4/6) that crosses the blood-brain barrier and demonstrates in vivo activity against intracranial human brain tumor xenografts. Mol Cancer Ther, 2011, 10:B234–B234.
2. Shapiro G, Rosen LS, Tolcher AW, et al A first-in-human phase I study of the CDK4/6 inhibitor, LY2835219, for patients with advanced cancer, J Clin Oncol. 2013, 31:2500–2500.
3. Gebbia V, Valerio MR, Firenze A, Vigneri P. Abemaciclib: safety and effectiveness of a unique cyclin-dependent kinase inhibitor. Expert Opin Drug Saf, 2020, 19(8):945-954.
You may like
Related articles And Qustion
See also
Lastest Price from Abemaciclib manufacturers

US $1.10-9.90/kg2025-08-15
- CAS:
- 1231929-97-7
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 100kg

US $5.00-0.50/KG2025-06-05
- CAS:
- 1231929-97-7
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS