Ethyl Oleate: A Comprehensive Overview
Introduce
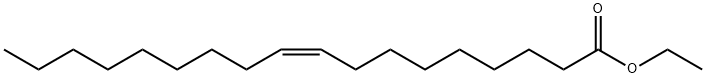
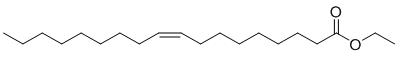
Ethyl Oleate, with the molecular formula C20H38O2, is a compound formed by esterification of oleic acid and ethanol. As an organic ester widely used in the fields of medicine, cosmetics, and industry, ethyl oleate has attracted much attention due to its unique physical and chemical properties and various uses. In the chemical and pharmaceutical industries, it is used to enhance the solubility and bioavailability of drugs, and as a preparation ingredient for various products.

Figure 1 Characteristics of Ethyl oleate
Nature
The physical and chemical properties of ETHYL OLEATE make it an ideal solvent in many industries. This compound is in a colorless to pale yellow liquid state at room temperature, with a weak aromatic odor and low viscosity. Its density is 0.87 g/cm ³, boiling point is about 216 ° C, flash point is around 113 ° C, and its chemical properties are relatively stable, making it less prone to oxidation reactions. This property gives it good safety when used under high temperature conditions.
Main components
ETHYL OLEATE is generated through esterification reaction of oleic acid and ethanol under the action of acid catalyst. Oleic acid is a naturally occurring monounsaturated fatty acid in animal and plant oils, with the chemical formula C18H34O2. Ethanol is a common organic solvent. The ethyl oleate formed by the reaction of the two contains a long-chain alkyl group (C18H34) and an ethyl ester group (- COOC2H5) in its chemical structure, giving it both the characteristics of long-chain fatty acids in oleic acid and the esterification properties derived from ethanol.
Due to the fact that the main components of ETHYL OLEATE are derived from natural raw materials and the esterification process is relatively simple, it has good biodegradability and environmental characteristics. Therefore, it is often used in applications that require environmental protection and safety, such as pharmaceutical preparations and food additives.
Purpose
Solubilizers and carriers in pharmaceutical formulations
ETHYL OLEATE is often used as a solubilizer for lipophilic drugs, especially in the preparation of injectable oily solutions. For example, it can significantly increase the solubility and bioavailability of steroid drugs (such as testosterone and other steroid drugs) when preparing injection solutions, reducing the irritation at the injection site. In addition, due to its good tissue compatibility and low toxicity, ETHYL OLEATE can also serve as a drug carrier to help control the release rate of drugs.
Lubricants and excipients in cosmetics
In the cosmetics industry, ETHYL OLEATE, as a lubricant and softener, is widely used in lotion, skin care oil, lipstick and other products. It can endow products with good extensibility and lubrication, while its natural source makes it a gentle and easily absorbable raw material for the skin. In high-end skincare products, ETHYL OLEATE is also used to improve the texture of the formula, giving the product a silky touch.
Lubricants and plastic additives in the industrial field
In industrial applications, ETHYL OLEATE is also commonly used as a lubricant, release agent, and plastic additive. Its chemical stability makes it perform well under high temperature and high pressure conditions, making it suitable for precision machinery, injection molding, rubber processing, and other fields. In addition, as an environmentally friendly solvent, ETHYL OLEATE also plays a role as an enhancer in the preparation process of certain biodiesel, helping to improve the lubrication performance and combustion efficiency of the fuel.
Storage method
Although ETHYL OLEATE has relatively stable chemical properties, its storage still needs to pay attention to the following points to maintain its physical and chemical properties:
Sealed storage
ETHYL OLEATE should be stored in a sealed container to avoid prolonged contact with air and prevent quality degradation due to oxidation. Sealed storage conditions help extend its lifespan.
Avoid light and ambient temperature environment
Although ETHYL OLEATE has good stability to light, long-term exposure to strong light may cause color changes or partial degradation. Therefore, it is recommended to store it in a cool and dark environment. The most suitable storage temperature is room temperature to avoid volatile loss caused by excessive temperature.
References:
[1] G. SARAVACOS G R S Marousis. Effect of ethyl oleate on the rate of air-drying of foods[J]. Journal of Food Engineering, 1988, 7 1: 263-270. DOI:10.1016/0260-8774(88)90008-8.[2] S. HAZARIKA . Ethyl oleate synthesis by Porcine pancreatic lipase in organic solvents[J]. Chemical Engineering Journal, 2002, 85 1: 1-98. DOI:10.1016/S1385-8947(01)00144-9.
Related articles And Qustion
Lastest Price from Ethyl oleate manufacturers

US $150.00/kg2025-11-24
- CAS:
- 111-62-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $9.10/KG2025-06-10
- CAS:
- 111-62-6
- Min. Order:
- 1KG
- Purity:
- 99.%
- Supply Ability:
- 10 ton