Introduction, synthesis and application of ethyl oleate
General description
Ethyl oleate is a colourless liquid that is normally formed by condensing ethanol and oleic acid. Notably, the compound is normally produced by the body during intoxication of ethanol. Its other names are 9-Octadecenoic acid (Z)-, Ethyl cis-9-octadecenoate, (Z)-9-Octadecenoic acid ethyl ester, and Oleic acid, ethyl ester [1]. The compound contributed to approximately 17% of the total fatty acids esterified to phosphatidylcholine in porcine platelets. Ethyl oleate is neutral and is a more lipid-soluble form of oleic acid. The compound is one of the fatty acid ethyl esters that is generated after the breakdown of ethanol in the body [2]. Moreover, ethyl oleate usually acts as a toxic mediator of ethanol in the heart, liver, pancreas, and brain.
Ethyl oleate is one of the fatty acid ethyl esters (FAEE) that is formed in the body after ingestion of ethanol. There is a growing body of research literature that implicates FAEEs such as ethyl oleate as the toxic mediators of ethanol in the body (pancreas, liver, heart, and brain). Among the speculations is that ethyl oleate may be the toxic mediator of alcohol in fetal alcohol syndrome [3]. The oral ingestion of ethyl oleate has been carefully studied and due to rapid degradation in the digestive tract it appears safe for oral ingestion. Ethyl oleate is not currently approved by the U.S. Food and Drug Administration for any injectable use. However, it is used by compounding pharmacies as a vehicle for intramuscular drug delivery, in some cases to prepare the daily doses of progesterone in support of pregnancy [4]. Studies which document the safe use of ethyl oleate in pregnancy for both the mother and the fetus have never been performed.
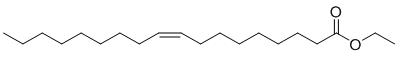
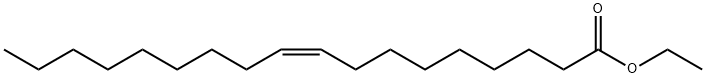
Fig.1 The structure of Ethyl oleate.
Synthesis

Fig.2 The synthetic route of Ethyl oleate [5].
Esterification and Transesterification Experiments. For esterification reactions, oleic acid (NF/FCC grade), hexane (GC resolv grade), and product (> = 98%) were obtained from Fischer Scientific. Ethanol (EtOH, 99.5%, anhydrous, 200 proof) was purchased from Acros Organics. The catalyst Amberlyst ® 15 (hydrogen form, strongly acidic, cation exchanger, dry, moisture ~ 5%) was purchased from Sigma-Aldrich. The reactor was heated to 65°C and was charged sequentially with oleic acid (0.5 mol), ethanol (1.0 mol), and catalyst (5% w/w). Once all components were charged into the reactor, aliquots (200 μL) were sampled periodically from the sample port. Then, hexane (2 mL as an internal standard for gas chromatography analysis) was added to the sample and mixed by a vortex mixer. 1 μL analyte was injected into a HP 6890 series gas chromatograph (FID detector, capillary column CP-Sil 88, 100 m length, 0.25 mm i.d., oven temperature 250°C, column temperature 181°C; J&W Scientific) for analysis. During pervaporation experiments, the permeate was collected in a cold trap over the entire duration of an individual experiment and was analyzed at the end of each experiment. The control experiments were carried out in the apparatus without the membrane and under the same conditions as pervaporation experiments to determine the contribution of pervaporation to the completion of the reaction. Ethyl oleate, Yield: 95%, Time: 60 h.
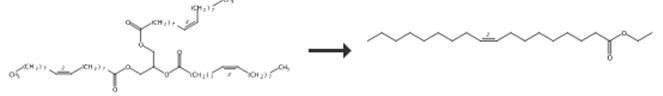
Fig.3 The synthetic route of Ethyl oleate [6].
Preparation of ethyl esters of oleic sunflower oil (1) formula: 604.8 g of oleic sunflower oil (OSO)(ITERG, M = 884.82 g.mol-1 - water content = 0.35% by weight) were placed in a jacketed reactor with 188.2 g of absolute ethanol. The whole was mixed with stirring at 650 rpm and heated to 65°C. 6.7211 g of NaOMe were then added to the reactor and a colour change of the product and the appearance of immediate cloudiness were then observed. The whole was then left to react for 1 hour at 70°C. The resulting reaction mixture was then transferred into a separating funnel in order to remove the glycerol and to evaporate off the ethanol. Neutralization was then performed with a few drops of HCl, followed by washing with water to neutrality. Finally, the residual water was distilled off on a rotavapor. 32.1 g of ethyl ester of sunflower oil (1), with a water content of 0.35% by weight, were thus obtained. According to the characterization performed by gas chromatography, a composition comprising 98.2% by weight of ethyl ester was obtained.
Chemical properties
Ethyl oleate occurs as a pale yellow to almost colorless, mobile, oily liquid with a taste resembling that of olive oil and a slight, but not rancid odor. Ethyl oleate is described in the USP32–NF27 as consisting of esters of ethyl alcohol and high molecular weight fatty acids, principally oleic acid. A suitable antioxidant may be included [7-8].
Pharmaceutical applications
Ethyl oleate is primarily used as a vehicle in certain parenteral preparations intended for intramuscular administration. It has also been used as a solvent for drugs formulated as biodegradable capsules for subdermal implantation) and in the preparation of microemulsions containing cyclosporinand norcantharidin. Microemulsion formulations containing ethyl oleate have also been proposed for topical and ocular delivery, and for liver targeting following parenteral administration [9]. Ethyl oleate has been used in topical gel formulations, and in self-microemulsifying drug delivery systems for oral administration. Ethyl oleate is a suitable solvent for steroids and other lipophilic drugs. Its properties are similar to those of almond oil and peanut oil. However, it has the advantage that it is less viscous than fixed oils and is more rapidly absorbed by body tissues. Ethyl oleate has also been evaluated as a vehicle for subcutaneous injection [10].
Uses
(1) Pharmaceutical Industry
Ethyl oleate is utilized as an ingredient for the preparations of pharmaceutical drugs that involves lipophilic substances such as steroids. Due to its quick degradation of the digestive system, ethyl oleate is employed as a way for intramuscular drug delivery by compounding pharmacies. In some cases, the compound is used in the preparation of day-to-day doses of progesterone in the sustenance of pregnancy [11].
(2) Transport Industry
It is used in the transport industry as a lubricant and as a plasticiser. It is also used as a planting agent and for treating surfaces [12].
(3) Food Industry
Ethyl oleate is used as a food additive and is regulated by the Food and Drug Administration. It is also used as a flavouring agent in food. Moreover, ethyl oleate is used as a solvent for pharmaceutical drug preparations involving lipophilic substances such as steroids [13]. It also finds use as a lubricant and a plasticizer. Ethyl oleate is regulated as a food additive by the Food and Drug Administration under "Food Additives Permitted for Direct Addition to Food for Human Consumption", 21CFR172.515.
Storage
Ethyl oleate should be stored in a cool, dry place in a small, wellfilled, well-closed container, protected from light. When a partially filled container is used, the air should be replaced by nitrogen or another inert gas. Ethyl oleate oxidizes on exposure to air, resulting in an increase in the peroxide value [14]. It remains clear at 5°C, but darkens in color on standing. Antioxidants are frequently used to extend the shelf life of ethyl oleate. Protection from oxidation for over 2 years has been achieved by storage in amber glass bottles with the addition of combinations of propyl gallate, butylated hydroxyanisole, butylated hydroxytoluene, and citric or ascorbic acid [15-17]. A concentration of 0.03% w/v of a mixture of propyl gallate (37.5%), butylated hydroxytoluene (37.5%), and butylated hydroxyanisole (25%) was found to be the best antioxidant for ethyl oleate. Ethyl oleate may be sterilized by heating at 150°C for 1 hour.
References
[1] C. Liu, Q. Liu, J. Zhang, C. Zeng, T. Zhang, Condensation-type waterproof potting composition and preparation method, Shenzhen Opute Industrial Material Co., Ltd., Peop. Rep. China . 2022, p. 7pp.
[2] T. Miyoshi, A. Sato, Multi-agent type hair treatment agent containing oils, cationic surfactant and higher alcohol, KOSE Corp., Japan . 2022, p. 22pp.
[3] H.P. Bhagwatwar, M. Nerurkar, Stable antiemetic emulsions, Steps BioSciences, Inc., USA . 2022, p. 11pp.
[4] L. Yang, S. Yang, Q. Lin, Q. Huang, H. Zhai, P. Wang, J. Ren, Glibenclamide pharmaceutical composition and preparation method therefor, Jiangsu Simcere Pharmaceutical Co., Ltd., Peop. Rep. China; Hainan Simcere Pharmaceutical Co., Ltd. . 2022, p. 19pp.
[5] Z. Zhang, Q. Liu, H. Wu, E. Huang, Compound amoxicillin colistin sulfate suspension injection and preparation method thereof, Sichuan Hengtong Animal Health Biotechnology Co., Ltd., Peop. Rep. China . 2022, p. 9pp.
[6] C. Florek, E. Cozzone, D.A. Armbruster, Biocompatible organogel matrices for preparation of a drug delivery depot, Depuy Synthes Products, Inc., USA . 2022, p. 81pp.
[7] M. Yi, Self-microemulsion composition of axitinib, Hunan Huize Bio-Pharmaceutical Co., Ltd., Peop. Rep. China . 2022, p. 29pp.
[8] Y. Wang, X. Sun, M. Yu, F. Wang, H. Yu, Preparation method of high-efficiency penetration conductive type pine trunk injection agent containing SAA-32, and its application in preventing and treating nematodiasis, Hangzhou Yisenjian Biotechnology Co., Ltd., Peop. Rep. China . 2022, p. 8pp.
[9] C. Feng, F. Pu, H. Li, G. Xu, J. Tian, C. Liu, H. Wu, W. Huang, G. Lin, H. Liu, Coolant for double mechanical seal and its preparation method, Hainan Handi Sunshine Petrochemical Co., Ltd., Peop. Rep. China . 2022, p. 7pp.
[10] Z. Li, B. Sundara Sekar, B.R. Lukito, Bioproduction of enantiopure (R)- and (S)-2-phenylglycinol from styrene and renewable feedstocks via artificial enzyme cascade, National University of Singapore, Singapore . 2022, p. 67pp.
[11] F. Shen, S. Zhu, H. Nie, Y. Hu, H. Jiang, Oral emulsion of terpene pharmaceutical composition and preparation method and application thereof, Beijing GrandPharma Johamu Pharmaceutical Co., Ltd., Peop. Rep. China . 2022, p. 12pp.
[12] W. Wang, H. Wang, X. Wang, J. Yang, Small-molecule bio-acid antiviral and antibacterial oil-soluble preparation, and its preparation method and application in antiviral and antibacterial drugs, Gansu Yanmei Pharmaceutical Co., Ltd., Peop. Rep. China . 2022, p. 13pp.
[13] Y. Fujita, T. Sakai, Cleaning agent composition and cleaning method, Kao Corporation, Japan . 2022, p. 99pp.
[14] A. Morimitsu, A. Umesawa, Polyurethane-containing two-component curable compositions as road surface repair agents, Auto Chemical Industry Co., Ltd., Japan . 2022, p. 23pp.
[15] R. Huang, Z. Ju, J. Luo, Biomarker for diagnosing or preventing liver injury, and test kit and application thereof, Central South University, Peop. Rep. China . 2022, p. 26pp.
[16] X. Jalenques, F. Bouchard, C. Barba, Deodorant anhydrous oily composition for dry purpose and fresh sprayable without propellant gas, L'Oreal, Fr. . 2022, p. 34pp.
[17] Z.-s. Tu, H.-w. Luo, Z.-y. Tan, J.-s. Cen, Mechanism of Astragalus-Taxillus-Chaenomeles medicine on treatment of osteoarthritis based on molecular docking and network pharmacology, Zhongguo Laonianxue Zazhi 41(24) (2021) 5585-5592.
Related articles And Qustion
Lastest Price from Ethyl oleate manufacturers

US $150.00/kg2025-11-24
- CAS:
- 111-62-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $9.10/KG2025-06-10
- CAS:
- 111-62-6
- Min. Order:
- 1KG
- Purity:
- 99.%
- Supply Ability:
- 10 ton