Uses and Preparation of (S)-(+)-3-Hydroxytetrahydrofuran
General description[1]
(S)-(+)-3-Hydroxytetrahydrofuran is a colorless to pale yellow transparent liquid with the formula C4H8O2 , which can be used as a detection reagent for chemiluminescence analysis. Its weight is 201.26 and CAS number is 86087-23-2 . At present, its market price is 1.7 million/ton, and there are more than 100 manufacturers in China alone, which has a high market value. Compared with (R)-3-hydroxytetrahydrofuran, many drug structures contain (S)-(+)-3-Hydroxytetrahydrofuran, and its price is low, so it is often used as an intermediate.
USE
(S)-(+)-3-Hydroxytetrahydrofuran (1) is an important pharmaceutical and chemical intermediate, which is widely used in anticancer drugs, hypoglycemic drugs, AIDS drugs and other drugs [2], such as the synthesis of antiarrhythmic drug ticardipine (2), hypoglycemic drug englizin (3), anti AIDS drug enronavir (4), anticancer drug afatinib (5), etc (Figure 1).
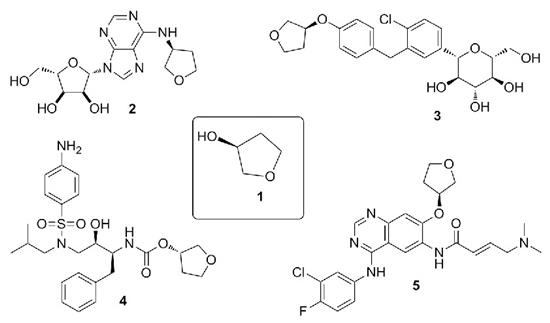
Figure 1. (S)-(+)-3-Hydroxytetrahydrofuran as a pharmaceutical intermediate
Since (S)-(+)-3-Hydroxytetrahydrofuran is contained in most of the drug structures and is cheap, it is often used as an intermediate. However, the direct use of 3-hydroxytetrahydrofuran racemate to prepare chiral drugs and intermediates has a high cost in the late resolution process, which is not conducive to industrial production. Therefore, in industrial production, (S)-(+)-3-Hydroxytetrahydrofuran is valued for its unique advantages such as price and chirality. In addition, in terms of pesticides, the introduction of (S)-(+)-3-Hydroxytetrahydrofuran group significantly improved the herbicidal activity and selectivity of diphenyl ether herbicides. Moreover, (S)-(+)-3-Hydroxytetrahydrofuran can also be used as an important intermediate in the synthesis of drugs for the treatment of thrombocytopenia and other diseases [3].
Preparation
The synthetic methods of chiral 3-hydroxytetrahydrofuran have been widely reported. A synthetic route of chiral 3-hydroxytetrahydrofuran was first proposed in 1983. This route takes chiral malic acid as the starting material and requires a large amount of lithium aluminum hydride, which is not suitable for large-scale production. At present, the preparation methods of (S)-(+)-3-Hydroxytetrahydrofuran mainly include chiral substrate synthesis, chiral catalyst asymmetric synthesis and enzyme catalyzed asymmetric synthesis [4-7].
Chiral substrate synthesis
1. L-malic acid and derivatives as raw materials
Using dimethyl L-malate as raw material, dimethyl (s)-2-tert-butyloxybutyrate was first protected by isobutene, and then reduced to (s)-2-tert-butyloxybutanol with cheap NaBH4 as reductant. Finally, under the catalysis of p-toluene sulfonic acid, the product (S)-(+)-3-Hydroxytetrahydrofuran was obtained through 2-position deprotection, dehydration and cyclization, with a yield of 65% (Scheme 1). This route uses cheap and easily available NaBH4 as reductant, which greatly reduces the cost of raw materials. The protection of dimethyl L-malate before reduction not only avoids racemization from the source, but also skillfully avoids the complexion of its 2-hydroxyl group with boron reagent, so that the reduced product is easy to separate and purify.
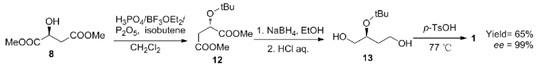
Scheme 1. Synthesis of (S)-(+)-3-Hydroxytetrahydrofuran by Dimethyl L-malate
2. (S)-4-chloro-3-hydroxybutyric acid ethyl ester as raw material
(S)-4-chloro-3-hydroxybutyric acid ethyl is an important raw material for the synthesis of (S)-(+)-3-Hydroxytetrahydrofuran, which can be obtained by stereoselective reduction of ethyl 4-chloro-3-butanoic acid. Using it as the starting material, the 2-hydroxy group was protected by isobutene to obtain ethyl (s)- 4-chloro-3-tert-butyloxybutyrate, and then reduced by NaBH4 to obtain (s)-4-chloro-3-tert-butyloxybutanol. Then cyclized under alkaline conditions to obtain (s)-3-tert-butyloxytetrahydrofuran, and finally deprotected by HCl gas to obtain (S)-(+)-3-Hydroxytetrahydrofuran, with an overall yield of 85% (Scheme 2). The synthetic method is cyclized first and then deprotected, which makes it easier to extract the product from the reaction solution and greatly improves the yield.
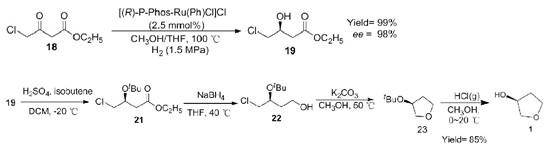
Scheme 2. Synthesis of 1 by (S)-4-chloro-3-hydroxybutyric acid ethyl
3. 1-butene-4-alcohol as raw material
Using 1-butene-4-alcohol as raw material, 3-butenyl-4-methylbenzene sulfonate was obtained by protecting hydroxyl with p-toluenesulfonyl chloride, then 2-(ethylene oxide-2-yl) ethyl-4-methylbenzene sulfonate was obtained by m-chloroperoxybenzoic acid (mCPBA) epoxidation, and then cyclized to obtain product 1, with a yield of 48%, and the yield of by-product (R) -2- (ethylene oxide-2-yl) ethyl-4-methylbenzene sulfonate was 47%. In addition, (S)-(+)-3-Hydroxytetrahydrofuran can also be directly obtained by Sharpless epoxidation from 1-butene-4-alcohol, with a yield of 95%. The greatest advantages of this route are short steps and mild reaction conditions, but the reagent cost is high (Scheme 3).
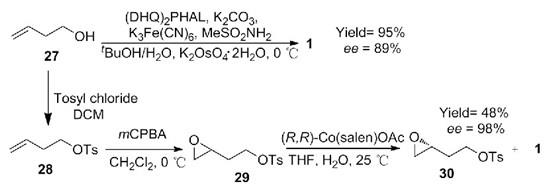
Scheme 3. Synthesis of 1 by 1-butene-4-alcohol
4. (S)-carnitine as raw material
(S)-carnitine was reduced by NaBH4 under ice bath to obtain (S)-2,4-dihydroxy-N,N,N-trimethylbutylamine base, then salify with hydrochloric acid to obtain (S)-2,4 -Dihydroxy-N,N,N-trimethylbutylamine chloride, and finally ring-closed at high temperature under alkaline conditions to obtain 1 with a total yield of 60% (Scheme 4). The raw materials of this route are cheap and easy to obtain, and the reaction steps are short, except that DMSO is used as the solvent in the last step, and the post-processing is troublesome.
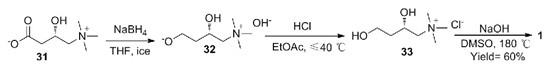
Scheme 4. Synthesis of 1 by (S)-carnitine
Chiral catalyst asymmetric synthesis
Chiral secondary alcohols can be synthesized by asymmetric borohydride reaction. Asymmetric borohydride reduction of 2,3-dihydrofuran can be achieved by using chiral boron catalyst 2,3-dihydrofuran. The yield of (S)-(+)-3-Hydroxytetrahydrofuran can reach 92%, and the EE value is 100% (Scheme 5). This method realizes the efficient synthesis of chiral compound 1, and the reaction can reach the level of 0.25 mol, which has a potential industrial application prospect.
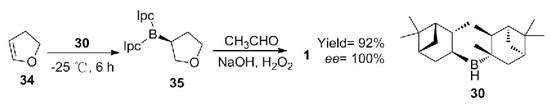
Scheme 5. Synthesis of 1 by Chiral catalyst asymmetric
Enzyme catalyzed asymmetric synthesis
The 3-hydroxytetrahydrofuran was first protected with acetyl chloride to obtain the esterification product 3-acetoxytetrahydrofuran, and then a highly enantioselective carbonatase (Bste E) was screened from the hydrolase, which was subjected to asymmet
Related articles And Qustion
Lastest Price from (S)-(+)-3-Hydroxytetrahydrofuran manufacturers

US $5.00-0.50/KG2025-06-07
- CAS:
- 86087-23-2
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $0.00/KG2025-04-21
- CAS:
- 86087-23-2
- Min. Order:
- 1KG
- Purity:
- 99% GC
- Supply Ability:
- 100kg