Is ethyl oleate safe for injection?
Introduction
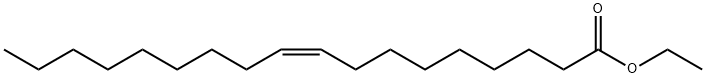
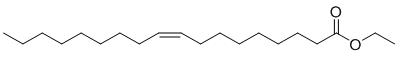
Ethyl oleate (EO) (9-octadecenoic acid ethyl ester; CAS# 111-62-6) is the ethyl ester of oleic acid. Oleic acid is the most widely distributed and the most extensively produced of all fatty acids in nature. In food, fatty acid esters of glycerol (triacylglycerols) make up the bulk of dietary fat and provide up to 40% of the daily caloric intake of man. Through the actions of lipases, both gastric and pancreatic, dietary triacylglycerols are hydrolyzed to free fatty acids and 2-monoacylglycerol. A complete hydrolysis to free glycerol also occurs. Following hydrolysis, the free fatty acids are absorbed and resynthesized into triacylglycerol within the intestinal mucosa. In vivo studies have demonstrated that EO and other fatty acid esters are rapidly hydrolyzed to ethanol and free fatty acid. The free fatty acid is then available for absorption and resynthesized into triacylglycerol.
It is a colourless liquid normally formed by condensing ethanol and oleic acid. Notably, the compound is normally produced by the body during the intoxication of ethanol. The compound contributed approximately 17% of the total fatty acids esterified to phosphatidylcholine in porcine platelets. Ethyl oleate is neutral and is a more lipid-soluble form of oleic acid. The compound is one of the fatty acid ethyl esters generated after the breakdown of ethanol in the body. Moreover, ethyl oleate is a toxic mediator of ethanol in the heart, liver, pancreas, and brain.
Uses
Ethyl oleate is generally used as a lipid carrier in positively charged SEDS, while oleylamine is generally used as a charge inducer. It falls under the category of GRAS and induces a charge of 30–35 mV. Besides oleyamine, chitosan and stearyl amine have also been reported as charge inducers. It is also found to be used as a lubricant and a plasticizer[1].
Ethyl oleate is primarily used as a vehicle in specific parenteral preparations intended for intramuscular administration. lt has also been used as a solvent for drugs formulated as biodegradable capsules for subdermal implantation) Moreover, in the preparation of microemulsions containing cyclosporin and norcantharidin.
Microemulsion formulations containing ethyl oleate have also been proposed for topical and ocular delivery and liver targeting following parenteral administration. Ethyl oleate has been used in topical gel formulations and self-micro-emulsifying drug delivery systems for oral administration. Ethyl oleate is a suitable solvent for steroids and other lipophilic drugs. Its properties are similar to those of almond oil and peanut oil. However, it has the advantage that it is less viscous than fixed oils and is more rapidly absorbed by body tissues. Ethyl oleate has also been evaluated as a vehicle for subcutaneous injection.
Safety
The absorption, distribution, and excretion of radiolabeled ethyl oleate (EO) was studied in Sprague–Dawley rats after a single, peroral dose of 1.7 or 3.4 g/kg body weight and was compared with a radiolabeled triacylglycerol (TG) containing only oleic acid as the fatty acid (triolein). Both test materials were well absorbed, with approximately 70–90% of the EO dose absorbed and approximately 90–100% of the TGdose absorbed[2]. At sacrifice (72 h post-dose), tissue distribution of EO-derived radioactivity and TG-derived radioactivity was similar. The tissue with the highest concentration of radioactivity in both groups was mesenteric fat. The other organs and tissues had very low concentrations of test material-derived radioactivity. Both test materials were rapidly and extensively excreted as CO2, with no remarkable differences between their excretion profiles. Approximately 40–70% of the administered dose for both groups was excreted as CO2 within the first 12 h (consistent with b-oxidation of fatty acids). Fecal elimination of EO appeared to be dose-dependent. At 1.7 g/kg, 7–8% of the administered dose was eliminated in the feces. At the dose of 3.4 g/kg, approximately 20% of the administered dose was excreted in the feces. Excretion of TG-derived radiolabel in the feces was approximately 2–4% for both doses. Overall, the results demonstrate that the absorption, distribution, and excretion of radiolabeled EO is similar to that of TG, providing evidence that the oleic acid moiety of EO is utilized in the body as a normal dietary TG-derived fatty acid.
To confirm the expected safety of EO in humans, a total of 235 subjects participated in a 12-week trial where two levels of ethyl oleate in a milk-based beverage were investigated: 8 g/day in a single serving (approximately 0.1 g/kg) and 16 g/day taken in two divided servings (approximately 0.2 g/kg). Adverse events (AEs) were recorded throughout the 12-week trial. In addition, a brief physical exam (including vital signs and body weight), ECGs, fasting serum chemistry profile, serum lipid profile, and urinalysis were performed at baseline and after study completion. Results showed that the incidence of reported AEs was similar between the EO and control groups. Analysis of comprehensive laboratory data revealed no EO exposure-related, clinically significant adverse changes in laboratory parameters. These studies demonstrated that EO has a highly favourable safety profile and is well tolerated in the diet.
References
[1] Robert C Bookstaff . “The safety of the use of ethyl oleate in food is supported by metabolism data in rats and clinical safety data in humans.” Regulatory Toxicology and Pharmacology 37 1 (2003): Pages 133-148.
[2] Garg, V. et al. “Application of self-emulsifying delivery systems for effective delivery of nutraceuticals." Emulsions (2016): 479-518.
You may like
Related articles And Qustion
See also
Lastest Price from Ethyl oleate manufacturers

US $150.00/kg2025-11-24
- CAS:
- 111-62-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $9.10/KG2025-06-10
- CAS:
- 111-62-6
- Min. Order:
- 1KG
- Purity:
- 99.%
- Supply Ability:
- 10 ton