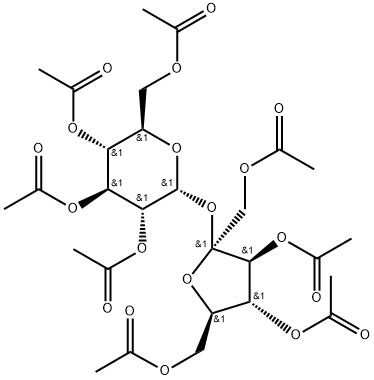
Sucrose octaacetate: Chemical property and uses
Introduction
Sucrose octaacetate (SOA) is a United States National Formulary (NF) monograph compendial material that has eight acetate groups attached to a sucrose moiety[1]. It is a natural product that has been extracted from the seeds of Annona cornifolia.
Chemical property
Sucrose octaacetate is only slightly soluble in water (sources give 0.25 to 1.4 g/L at room temperature) but is soluble in many common organic solvents, such as toluene and ethanol, from which it can be crystallized by evaporation. It is also soluble in supercritical carbon dioxide.
It is nontoxic and has several uses based on its bitter taste. For example, sugar is rendered too bitter and is eaten at a concentration of 0.06% (w/w) SOA. SOA can form 255 different possible isomers and degradation products with very low molar absorptivity. Its ultraviolet molar absorptivity at 210nm has been reported to be 439 absorption units/cm/M in water and 442 absorption units/cm/M in 30:70 acetonitrile water.
Uses

Sucrose octaacetate is an acetylated derivative of sucrose with an intensely bitter taste and can be used as a bitter-tasting surrogate. It is a natural product that has been isolated from the roots of several species of Clematis. The root of Clematis japonica contains 0.15% of this compound by dry weight. Presumably, this bitter-tasting compound discourages predation on these plants. Humans have used this chemical commercially as a gustatory repellant because of its bitter taste. It has been used to denatured alcohol, as a deterrent to fingernail biting, and to make sugar for animal feed inedible to humans. At a concentration of 0.06%, this compound makes sugar too bitter to eat. This compound has been shown to be nontoxic and does not affect the taste of the flesh of food animals or the milk of cows that consume it[2].
Besides being used as a food additive, it is also used as an adhesive and plasticizer. Sucrose octaacetate is also used in many pesticides, insecticides, and other toxic products as a deterrent to accidental poisoning. Sucrose octaacetate can also be used as an in situ seed and a soft template to synthesize polyaniline (PANI) nanofibers.
References
[1] Thierry D. Mann, William F. Wood, James D. Mosher. “Preparation of sucrose octaacetate—A bitter-tasting compound.” Journal of Chemical Education 69 8 (1992): 668.
[2] William Craig Stagner. “Sucrose octaacetate.” Profiles of drug substances, excipients, and related methodology 44 (2019): 267–291.
You may like
See also
Lastest Price from Sucrose octaacetate manufacturers
US $0.00-0.00/kg2025-11-22
- CAS:
- 126-14-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00/Kg/Drum2025-04-21
- CAS:
- 126-14-7
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 10000kgs