Deuterium oxide: Chemical structures, Reactions and Applications
Chemical structures of Deuterium oxide
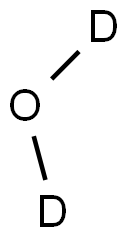
Deuterium oxide (D2O), also known as "heavy water", is a colourless liquid. There are two stable isotopes of hydrogen, protium (1H) and deuterium (2H; D). Heavy water consists of one oxygen atom (O) and two 2H atoms (D). Melting point 3.8 ºC, boiling point 101.4 ºC, miscible with water. Its structural formula is shown below:
Reactions
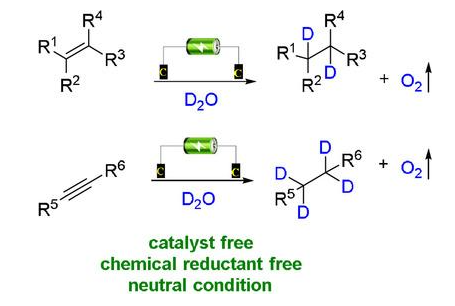
A method for electrochemical deuteration of α,β-unsaturated carbonyl compounds in the absence of catalysts and external reducing agents is reported, with deuteration rates as high as 99% in 2 h and yields as high as 91%. The use of graphite felts for both cathode and anode was the key to ensure chemoselectivity and high deuteration rates under neutral conditions without the need for external reducing agents. This approach offers a number of advantages over previously reported deuteration reactions using stoichiometric metal reductants. Mechanistic experiments have shown that oxygen evolution at the anode not only eliminates the need for an external reducing agent, but also modulates the pH of the reaction mixture, keeping it approximately neutral.
Applications
Deuterium oxide can be used in nuclear reactors to slow down neutrons so that they react with fissionable 235U instead of non-fissionable 238U, eliminating the need for uranium enrichment.
D2O is used in isotope tracing methods to study the moisture penetration of polymer encapsulants. The polymer encapsulant was placed into a temperature and humidity chamber (40°C, 90% RH) containing D2O. The deuterium signal of the samples was then analysed by time-of-flight secondary ion mass spectrometry (ToF-SIMS). The moisture penetration distance versus time was plotted. The results showed that in this work, the moisture penetration rate into the polymer encapsulation material was about 40 microns per hour.
D2O is also widely used to study the metabolism of drugs and toxic substances in humans and other animals. Deuterium oxide drugs often act differently from protonated drugs. Studies have shown that systemic administration of D2O also inhibits the growth and metastasis of malignant melanoma.
References:
[1] LOIS L, BISHENG W, XI Z, et al. Study of moisture penetration in polymer encapsulant by deuterium oxide (D20) isotope tracing technique[J]. 2022 IEEE 24th Electronics Packaging Technology Conference (EPTC), 2022. DOI:10.1109/eptc56328.2022.10013266.
[2] XU LIU. Chemical-Reductant-Free Electrochemical Deuteration Reaction using Deuterium Oxide[J]. Angewandte Chemie, 2020. DOI:10.1002/ange.202005765.
[3] JANA JANDOVA. Deuterium Oxide (D2O) Induces Early Stress Response Gene Expression and Impairs Growth and Metastasis of Experimental Malignant Melanoma.[J]. Cancers, 2021. DOI:10.3390/cancers13040605.
Related articles And Qustion
See also
Lastest Price from DEUTERIUM OXIDE manufacturers

US $0.00-0.00/KG2025-12-02
- CAS:
- 7789-20-0
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $15.00-10.00/KG2023-03-04
- CAS:
- 7789-20-0
- Min. Order:
- 1KG
- Purity:
- 99.9%
- Supply Ability:
- 10 Ton