Deuterium Oxide: From Nuclear Power Advancements to Therapeutic Potential in Health Research
Description
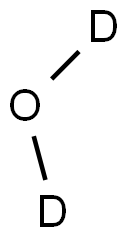
Deuterium oxide(D2O) is a form of water in which hydrogen atoms are all deuterium rather than the common hydrogen-1 isotope (1H, also called protium) that makes up most of the hydrogen in normal water.

Properties
Deuterium oxide is less dissociated at a given temperature, and it does not have the slightly blue color of regular water. It can taste slightly sweeter than regular water, though not to a significant degree. Heavy water affects biological systems by altering enzymes, hydrogen bonds, and cell division in eukaryotes.
Health and Research Implications
In the realm of health and science, deuterium oxide's impact is increasingly recognized. It is utilized as a solvent in nuclear magnetic resonance (NMR) spectroscopy for dissolving internal standards and samples. Beyond analytical uses, deuterium oxide has shown potential as a therapeutic agent, particularly in treatments targeting human pancreatic cancer. Additionally, it assists in determining total body water and enhancing the thermal stability of macromolecules, cells, and tissues. The growing number of patents related to deuterium oxide underscores its expanding role in medical research and potential therapeutic applications.
Applications
Neutron moderator
Deuterium oxide is used in certain types of nuclear reactors, where it acts as a neutron moderator to slow down neutrons so that they are more likely to react with the fissile uranium-235 than with uranium-238, which captures neutrons without fissioning. The CANDU reactor uses this design. Light water also acts as a moderator, but because light water absorbs more neutrons than heavy water, reactors using light water for a reactor moderator must use enriched uranium rather than natural uranium, otherwise criticality is impossible.
Infrared spectroscopy
Deuterium oxide is often used instead of water when collecting FTIR spectra of proteins in solution. H2O creates a strong band that overlaps with the amide I region of proteins. The band from D2O is shifted away from the amide I region.
You may like
Related articles And Qustion
Lastest Price from DEUTERIUM OXIDE manufacturers

US $0.00-0.00/KG2025-12-02
- CAS:
- 7789-20-0
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $15.00-10.00/KG2023-03-04
- CAS:
- 7789-20-0
- Min. Order:
- 1KG
- Purity:
- 99.9%
- Supply Ability:
- 10 Ton