Orlistat: Medical use, mechanism and side effects
General description
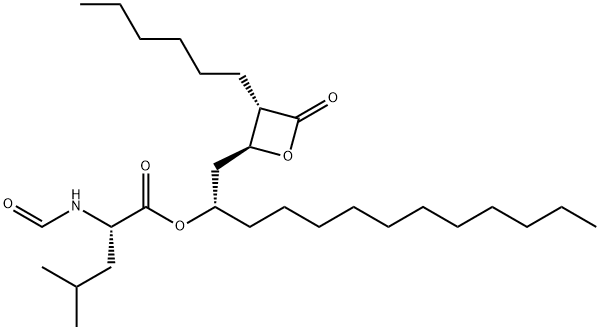
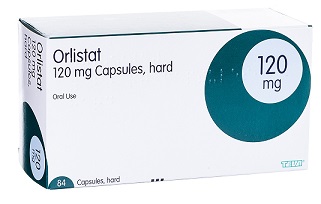
Orlistat is a drug designed to treat obesity. It is marketed as a prescription drug under the trade name Xenical by Roche in most countries, and is sold over-the-counter as Alli by GlaxoSmithKline in the United Kingdom and the United States.[1] Its primary function is preventing the absorption of fats from the human diet by acting as a lipase inhibitor, thereby reducing caloric intake. It is intended for use in conjunction with a healthcare provider-supervised reduced-calorie diet. Orlistat is the saturated derivative of lipstatin, a potent natural inhibitor of pancreatic lipases isolated from the bacterium Streptomyces toxytricini.[2] However, due to its relative simplicity and stability, orlistat was chosen over lipstatin for development as an anti-obesity drug. The effectiveness of orlistat in promoting weight loss is definite but modest. Pooled data from clinical trials suggest that people given orlistat in addition to lifestyle modifications, such as diet and exercise, lose about 2–3 kilograms (4.4–6.6 lb) more than those not taking the drug over the course of a year. Its appearance is as follows:

Figure 1 The appearance of Orlistat
Medical use
Orlistat is used for the treatment of obesity. The amount of weight loss achieved with orlistat varies. In one-year clinical trials, between 35.5% and 54.8% of subjects achieved a 5% or greater decrease in body mass, although not all of this mass was necessarily fat. Between 16.4% and 24.8% achieved at least a 10% decrease in body fat. After orlistat was stopped, a significant number of subjects regained weight—up to 35% of the weight they had lost. It reduces the incidence of diabetes type II in people who are obese around the same amount that lifestyle changes do. Long-term use of orlistat also leads to a very modest reduction in blood pressure (mean reductions of 2.5 and 1.9 mmHg in systolic and diastolic blood pressure respectively).[3]
Mechanism
Orlistat works by inhibiting gastric and pancreatic lipases, the enzymes that break down triglycerides in the intestine.[4] When lipase activity is blocked, triglycerides from the diet are not hydrolyzed into absorbable free fatty acids, and instead are excreted unchanged. Only trace amounts of orlistat are absorbed systemically; the primary effect is local lipase inhibition within the GI tract after an oral dose. The primary route of elimination is through the feces. Orlistat was also recently found to inhibit the thioesterase domain of fatty acid synthase (FAS), an enzyme involved in the proliferation of cancer cells but not normal cells. However, potential side effects of Orlistat, such as inhibition of other cellular off-targets or poor bioavailability, might hamper its application as an effective antitumor agent. One profiling study undertook a chemical proteomics approach to look for new cellular targets of orlistat, including its off-targets.[5] Orlistat also shows potential activity against the Trypanosoma brucei parasite.
Side effects
The primary side effects of the drug are gastrointestinal-related, and include steatorrhea (oily, loose stools with excessive flatus due to unabsorbed fats reaching the large intestine), fecal incontinence and frequent or urgent bowel movements. To minimize these effects, foods with high fat content should be avoided; the manufacturer advises consumers to follow a low-fat, reduced-calorie diet. Oily stools and flatulence can be controlled by reducing the dietary fat content to somewhere in the region of 15 grams per meal. The manual for Alli makes it clear that orlistat treatment involves aversion therapy, encouraging the user to associate eating fat with unpleasant treatment effects. Side effects are most severe when beginning therapy and may decrease in frequency with time; It has also been suggested that the decrease in side effects over time may be associated with long-term compliance with a low-fat diet.[6]
References
[1]Bodkin J, Humphries E, McLeod M (2003). The total synthesis of (?)-tetrahydrolipstatin. Australian Journal of Chemistry. 56 (8): 795–803.
[2]Barbier P, Schneider F (1987). Syntheses of tetrahydrolipstatin and absolute configuration of tetrahydrolipstatin and lipstatin. Helvetica Chimica Acta. 70 (1): 196–202.
[3]Siebenhofer, A; Jeitler, K; Horvath, K; Berghold, A; Posch, N; Meschik, J; Semlitsch, T (2 March 2016). Long-term effects of weight-reducing drugs in people with hypertension. The Cochrane Database of Systematic Reviews. 3: CD007654.
[4]Heck, A. M.; Yanovski, J. A.; Calis, K. A. (March 2000). Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 20 (3): 270–279.
[5]Peng-Yu, Yang (December 2009). Activity-Based Proteome Pro?ling of Potential CellularTargets of Orlistat -An FDA-Approved Drug with Anti-TumorActivities. Journal of the American Chemical Society. 132 (2): 656–66.
[6]Mancini MC, Halpern A (2006). Pharmacological treatment of obesity. Arq Bras Endocrinol Metabol. 50 (2): 377–89.
You may like
Related articles And Qustion
See also
Lastest Price from Orlistat manufacturers

US $1.00-4.00/KG2025-09-02
- CAS:
- 96829-58-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $0.00-0.00/mg2025-05-28
- CAS:
- 96829-58-2
- Min. Order:
- 10mg
- Purity:
- 99%+ HPLC
- Supply Ability:
- 1000