Exenatide: chemistry, pharmacokinetics and its toxicity
Introduction
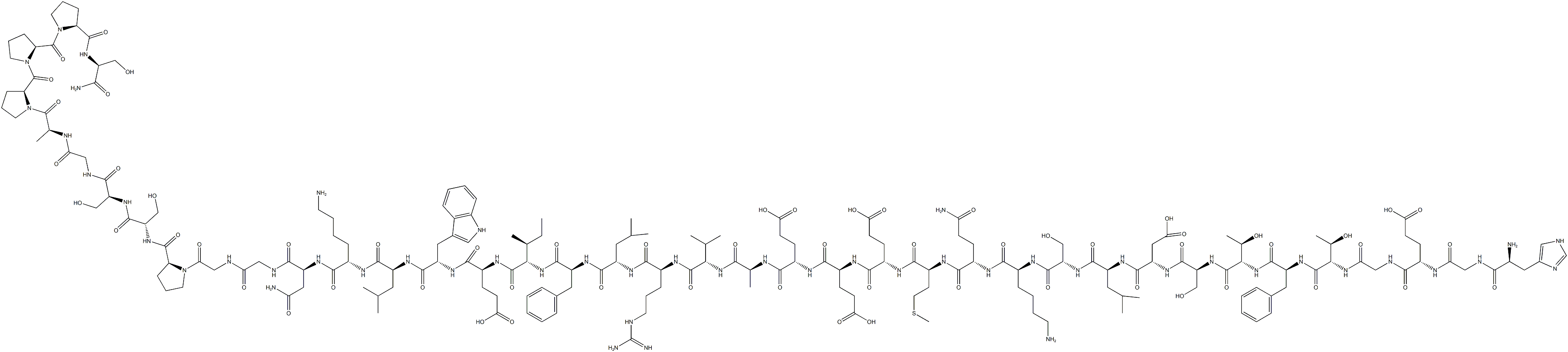
Exenatide(also known as Exendin-4;Figure 1), which belongs to the glucagon-like peptide-1 (GLP-1) receptor agonist family, has been used to treat diabetes and obesity chiefy owing to its prominent effects on insulin resistance, weight loss, and metabolic disorders. Some clinical randomized controlled trials (RCTs) have demonstrated that exenatide also has benefcial effects on pregnancy rate, the menstrual frequency ratio (MFR), insulin resistance (IR), and weight reduction in women who are suffering from PCOS. Whether exenatide is the preferred treatment option for patients with PCOS still needs to be confirmed by large-scale clinical studies and adequate evidence-based data. When choosing a drug regimen,it is necessary to consider efficacy and safety. The most common adverse reactions associated with exenatide treatment are gastrointestinal reactions. Other side effects include sclerosis at the injection site, headache,and allergic reactions.[1]

Chemistry of Exenatide
Exenatide has a molecular formula (C184H282N50O60S) and a molecular weight of 4187g/mol. It has an exact mass of 4185.0306624 g/mol and a monoisotopic mass of 4184.0273075 g/mol (National Center for Biotechnology Information-2023).Exenatide is a peptide that consists of 39 amino acids that originates from the salivary secretion of the Gila Monster (Heloderma suspectum). In the venom of H.suspectum, exenatide and GLP-1 are the products of separate genes specific to different tissues. This results in them being closely connected, yet they display distinguishing traits that set them apart.Additionally, the initial exploration of the medicinal possibilities of the synthetic version of exenatide,commonly referred to as exenatide, focused on its use in diabetes treatment. [2]
Pharmacokinetics of Exenatide
Animal models using various administration routes have been used to study the pharmacokinetics of exenatide.According to the administration route and provided dosage, the pharmacokinetics of exenatide in experimental animal models vary significantly. Exenatide is more bioavailable when administered subcutaneously (s.c.) compared to when taken orally. The reason for this is that when exenatide is taken orally, it is absorbed by the cells lining the intestines and enters the portal system of liver,where it can potentially be broken down by peptidase enzymes in the liver. The bioavailability of exenatide is lowered by the initial metabolic effects of oral consumption. However, in comparison with the s.c.route, when administered intravenously (i.v.), exendin4 exhibits a briefer half-life in animal models. Therefore, s.c. route is ideal for its administration in human volunteers. Exenatide QW and exenatide BID are the two formulations of exenatide that are now available for medicinal purposes. Exenatide QW is a prolonged-release, extended duration variant of exenatide whereas a quick-acting version of exenatide called exenatide BID is intended for twice-daily use. To achieve the prompt release of exenatide from the formulation, the absorption pattern of exenatide BID adheres to zero-order kinetics whereas exenatide QW displays a first-order rate during the absorption, indicating a quick initial release. In a population pharmacokinetics study with exenatide QW, 64 individuals were given either a single dosage of 2.5–10mg or several doses of 0.8 and 2mg over 15weeks. The results showed that exenatide remained at a constant level in diabetes patients for 8–10 weeks. Therefore, to maintain this active peptide's bioavailability, it is administered weekly. For exenatide, the main renal elimination mechanism is passive glomerular filtration. Exenatide's proteolytic effects are mediated by aminopeptidases, serine proteases along with metalloproteases. The in vitro studies also imply that exenatide is initially broken down in the liver by endopeptidases before being further eliminated by exopeptidases. Moreover, several strategies have been explored to enhance the bioavailability of exenatide for consistent delivery to β-cells. Interestingly, one such modification involves an exenatide analogue called E19. Exenatide can sometimes exhibit poor hydrophilicity, leading to the aggregate formation that cannot effectively interact with GLP-1 receptors, thus diminishing its bioavailability. On the contrary,the E19 analogue offers improved folding stability and water solubility which contributes to improved bioavailability.[2]
Safety and tolerability
Nausea
In early clinical trials with exenatide, nausea had been the most frequent treatment-emergent adverse event. This tended to occur most upon initiation of therapy and subsided over the first week. Overall, exenatide was generally well tolerated. There did not appear to be any evidence of a clear safety concern, but there were some important observations pertaining to tolerability in some patients. The most common treatment-emergent adverse event was dose-dependent nausea, and this was most notable at the time of initiating therapy and was reported at lower incidence thereafter.Nausea was mostly mild or moderate in intensity,with a low incidence of severe nausea (~6%) and low withdrawal from the study due to nausea (~3%). An increase in gastrointestinal side effects is also seen with other incretin mimetics and is thought to be a consequence of the effect of GLP-1on reducing gastric emptying.
Hypoglycemia
Mild to moderate hypoglycemia has been reported in phase II and III studies with exenatide. However, it appears that it is only when in combination with a sulfonylurea that the risk of hypoglycemia is increased. In the similarly designed phase III study in which exenatide was added to a background of metformin therapy,there was no increase in reported hypoglycemia,even though overall glycemia had improved. It is therefore speculated that the increase in hypoglycemia observed in some exenatide studies was probably caused by the background susceptibility to hypoglycemia often observed in sulfonylurea-treated patients, coupled with a lower ambient glycemia.
Overall, exenatide is generally well tolerated.No clinically relevant effects of exenatide treatment on clinical laboratory analyses, blood pressure orheart rate have been reported. Results from an open-label extension of the AMIGO trials inpatients who had completed 18 months of treatment have revealed no new safety or tolerability issues.[3]
References
[1] Hu Y, Song X, Hamiti S, et al. Comparison of exenatide alone or combined with metformin versus metformin in the treatment of polycystic ovaries: a systematic review and meta-analysis. BMC Endocr Disord. 2023;23(1):250. Published 2023 Nov 16. doi:10.1186/s12902-023-01497-x
[2]Verma A, Chaudhary S, Solanki K, Goyal A, Yadav HN. Exendin-4: A potential therapeutic strategy for Alzheimer's disease and Parkinson's disease. Chem Biol Drug Des. 2024;103(1):e14426. doi:10.1111/cbdd.14426
[3]Barnett AH. Exenatide. Drugs Today (Barc). 2005;41(9):563-578. doi:10.1358/dot.2005.41.9.893704
You may like
See also
Lastest Price from Exendin-4 manufacturers

US $1.00/g2025-04-21
- CAS:
- 141758-74-9
- Min. Order:
- 1g
- Purity:
- 97.0%-103.0%
- Supply Ability:
- 1kg/month

US $1000.00-400.00/g2025-04-21
- CAS:
- 141758-74-9
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 5000