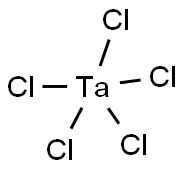
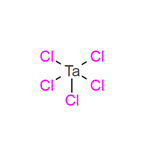
Tantalum(V) chloride: Preparation and Application
Tantalum(V) chloride is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. The physical properties of tantalum(V) chloride include its solubility, which increases slightly for the following series of aromatic hydrocarbons: benzene, toluene, and mesitylene. Tantalum(V) chloride is less soluble in cyclohexane and carbon tetrachloride than in the aromatic hydrocarbons. Such solutions of tantalum(V) chloride are also known to be poor conductors of electricity, indicating little ionization. TaCl5 is purified by sublimation to give white needles.

Preparation
There are multiple ways to synthesize tantalum(V) chloride. One method involves reacting powdered metallic tantalum with chlorine gas at between 170 and 250 °C. This reaction can also be performed using HCl at 400 °C. Another method involves reacting tantalum pentoxide and thionyl chloride at 240 °C. Tantalum(V) chloride is commercially available, but samples can be contaminated with tantalum(V) oxychloride (TaOCl3), formed by hydrolysis.
Application
Tantalum(V) chloride is used in the preparation of catalysts for the polycyclotrimerizations of alkenediynes, chloro-aryloxide compounds, and for the plasma-enhanced atomic layer deposition of tantalum nitride films. It acts as a starting material for new edge-bridged octahedral M6 cluster compounds. Tantalum(V) chloride is involved in the preparation of tantalum(V) oxychloride and tantalum pentoxide.
It is electrophilic and behaves like a Friedel–Crafts catalyst, similar to aluminum(III) chloride. It forms adducts with a variety of Lewis bases. It also reacts with phosphorus pentachloride and phosphorus oxychloride, the former as a chloride donor and the latter serves as a ligand, binding through the oxygen. Tantalum(V) chloride reacts with tertiary amines to give crystalline adducts .
Tantalum(V) chloride can undergo chloride displacement reactions. For instance, it reacts at room temperature with an excess of triphenylphosphine oxide to give oxychlorides. In the presence of ammonia as a HCl acceptor, all five chloride ligands are displaced with formation of Ta(OEt)5. Similarly, TaCl5 reacts with lithium methoxide in anhydrous methanol to form related methoxy derivatives.
In conclusion, tantalum(V) chloride is a white powder that is used as a starting material in tantalum chemistry. It can be synthesized by reacting powdered metallic tantalum with chlorine gas or by reacting tantalum pentoxide and thionyl chloride. Tantalum(V) chloride is involved in the preparation of catalysts, new edge-bridged octahedral M6 cluster compounds, tantalum(V) oxychloride, and tantalum pentoxide. It behaves like a Friedel–Crafts catalyst, forms adducts with Lewis bases, and undergoes chloride displacement reactions.
Related articles And Qustion
See also
Lastest Price from Tantalum(V) chloride manufacturers
US $0.00/kg2022-09-22
- CAS:
- 7721-01-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg