Tantalum(V) Chloride: Properties Applications in Organic Chemistry and Healthy Hazards
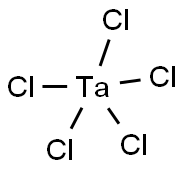
Tantalum(V) chloride is a common tantalum compound. The chemical formula of tantalum chloride is TaCl5. It is a yellow crystal with a strong pungent odor. It is insoluble in water, but can react with a variety of organic solvents. At high temperatures, tantalum chloride decomposes into tantalum chloride trimer and tantalum chloride monomer. It has stable chemical properties and can react with other compounds to form different compounds.

Figure 1. Tantalum(V) chloride
Chemical properties
Stability: The chemical properties are not very stable. It will decompose to form tantalic acid in humid air or water.
Structure: Tantalum(V) chloride is a dimer in the solid state, and two tantalum atoms are connected by two chlorine bridges. In the gaseous state, Tantalum(V) chloride is a monomer with a trigonal bipyramidal structure.
Reactivity: Tantalum(V) chloride is a strong Lewis acid and can react with Lewis bases to form adducts. It can react with a variety of compounds, such as ethers, phosphorus pentachloride, phosphorus oxychloride, tertiary amines, etc.
Preparation method
Tantalum(V) chloride reaction: Tantalum(V) chloride can be prepared by reacting powdered tantalum metal with chlorine at 170~250℃. This reaction can also be carried out with HCl at 400℃.
Tantalum pentoxide reacts with thionyl chloride: Tantalum(V) chloride can also be produced by reacting tantalum pentoxide with thionyl chloride at 240°C.
Uses
Chlorinating agent for organic compounds: Tantalum(V) chloride can be used as a chlorinating agent for organic compounds to promote chlorination reactions.
Chemical intermediates: In the chemical industry, Tantalum(V) chloride is used as a raw material for the preparation of ultra-high purity tantalum metal and chemical intermediates.
Preparation of tantalum: Metallic tantalum can be prepared by hydrogen reduction of Tantalum(V) chloride. This method includes depositing tantalum from the gas phase on a heated substrate carrier to produce a dense metal, or reducing tantalum chloride with hydrogen in a fluidized bed to produce spherical tantalum powder.
Other applications: Tantalum(V) chloride is also used as an intermediate for the preparation of optical glass and tantalum carbide, and as a raw material for the preparation of tantalates and rubidium tantalate in the electronics industry.
In addition, it is also used to manufacture dielectrics and is widely used in the preparation of surface polishing deburring and preservatives.
Related articles And Qustion
See also
Lastest Price from Tantalum(V) chloride manufacturers
US $0.00/kg2022-09-22
- CAS:
- 7721-01-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg