D-(-)-Salicin: Phytochemistry, Biosynthesis and Metabolism
D-(-)-Salicin is a pure natural active substance extracted from the branches or bark of the willow of the willow family by modern extraction technology.

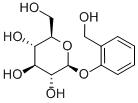
Figure 1. D-(-)-Salicin
Product Function
Treatment of fever, colds and infections
D-(-)-Salicin is used as a "natural aspirin" to treat minor fevers, colds, infections (flu), acute and chronic rheumatic discomfort, headaches and pain caused by inflammation. Aspirin (acetylsalicylic acid), as a synthetic alternative to D-(-)-Salicin, has potentially dangerous gastrointestinal side effects. In its natural form, D-(-)-Salicin can pass harmlessly through the gastrointestinal system and is converted to salicylic acid in the blood and liver. The conversion process takes several hours, so the body does not feel the effects immediately, but the general effect lasts for several hours.
Participate in redox reactions to relieve arthritis pain and back pain
D-(-)-Salicin is believed to be the source of the anti-inflammatory and analgesic ability of white willow bark. The analgesic ability of white willow bark is generally slower to take effect, but lasts longer than ordinary aspirin products. A trial found that an herbal compound containing 100 ng of D-(-)-Salicin improved pain relief in arthritis patients after taking it for two months. Another trial found that taking 1360 mg of white willow bark extract (containing 240 mg of D-(-)-Salicin) daily for two weeks was more effective in treating joint pain and/or arthritis. High doses of white willow bark extract may also help relieve back pain. A four-week trial found that white willow bark extract containing 240 mg of D-(-)-Salicin was effective in reducing the worsening of back pain.
Example of preparation of D-(-)-Salicin
1. Crushing: Crush 50 kg of white willow branches and leaves into small pieces of 1 to 2 cm. If the raw material is too large, the extraction is not thorough; if the raw material is too small, the residue is difficult to remove;
2. Hydrolysis extraction: add 2.5kg calcium oxide and 15kg water to the raw material, stir evenly, put into the extraction tank, hydrolyze for 3.5 hours, and hydrolyze the D-(-)-Salicin derivatives in the raw material; then add 200kg 80% ethanol to the extraction tank, reflux extraction for 80 minutes, filter, extract the residue twice, and combine the filtrate; vacuum concentrate the extract, control the temperature below 80℃, take the concentrated filtrate, and obtain 30kg of extract (i.e. semi-finished product) with a specific gravity of 1.1;
3. Refining steps:
(1) Extraction Take, dissolve the extract with 15kg water, add 15kg n-butanol to extract, separate the n-butanol phase and the water phase of the extract, add 15kg n-butanol to the water phase for extraction, repeat the extraction process twice, combine the n-butanol phase of the extract, and obtain 65kg;
(2) Crystallization, stop concentrating when the extract is concentrated to 17% of the original solution, and let it stand to obtain crude crystals;
(3) Recrystallization, heat and dissolve the above crude crystals with 0.2kg water and 3kg ethanol, filter, let the filtrate stand for 24 hours for recrystallization, and obtain 350g of crystals, with a content of 96.15% detected by HPLC.
You may like
Related articles And Qustion
Lastest Price from D-(-)-Salicin manufacturers

US $1200.00-1100.00/ton2025-10-15
- CAS:
- 138-52-3
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $690.00/g2025-06-01
- CAS:
- 138-52-3
- Min. Order:
- 1g
- Purity:
- 99 %
- Supply Ability:
- 5000 Kg