Baloxavir marboxil: mechanism of action, pharmacokinetics and side effects
General Description
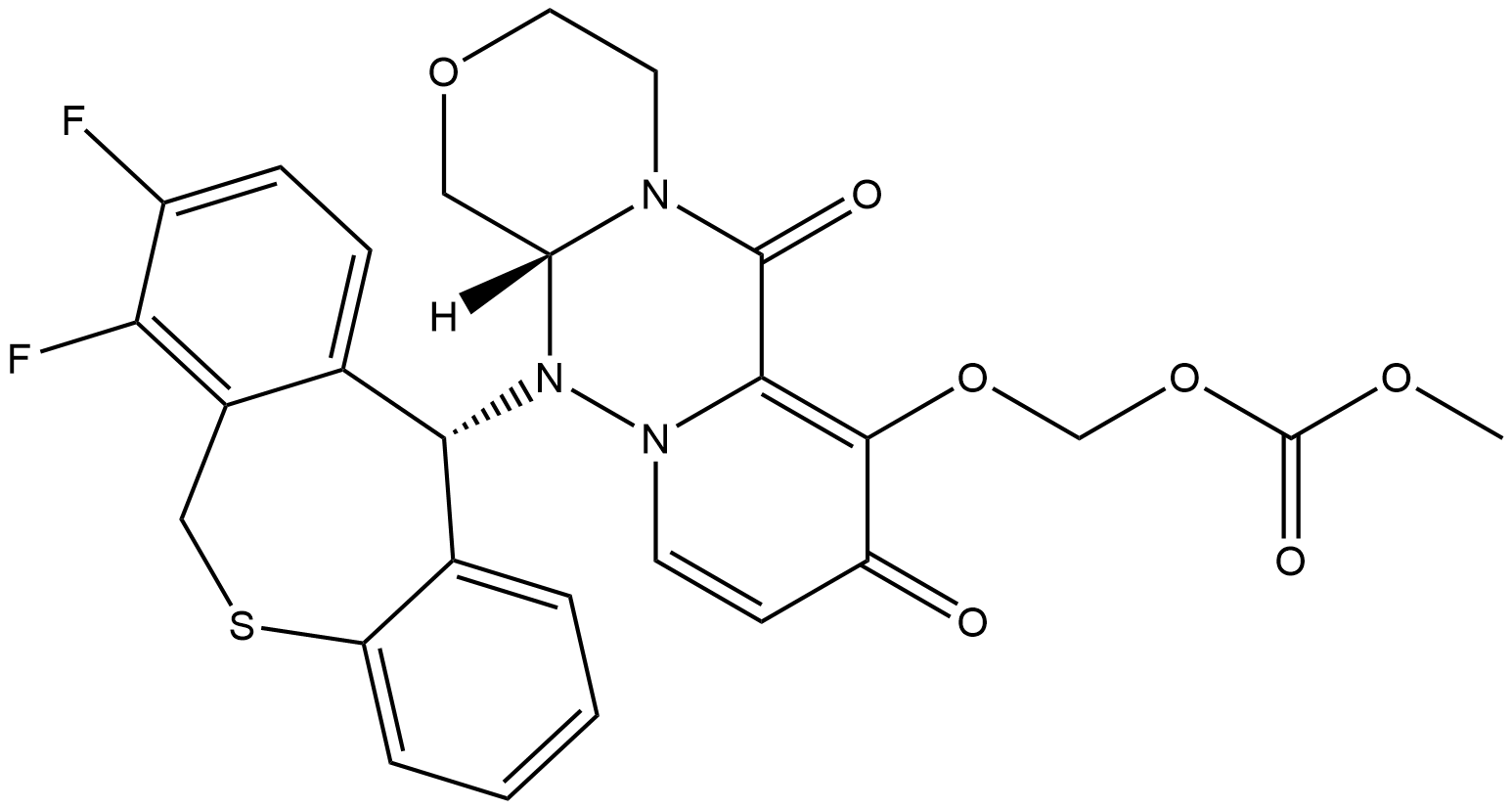
Baloxavir marboxil is a medication used to treat influenza infections. It works by inhibiting the endonuclease activity of the polymerase acidic protein (PA) in viral ribonucleoprotein complexes, preventing the replication of influenza viruses. The drug undergoes rapid hydrolysis in the body to form its active metabolite, baloxavir acid (BXA), which is primarily metabolized by UGT1A3 and excreted through feces and urine. Baloxavir marboxil has shown good tolerability with reversible side effects such as headache and mild perturbations in blood composition. Clinical studies have reported a low incidence of side effects compared to a placebo.
Figure 1. Tablet of baloxavir marboxil
Mechanism of action
Baloxavir marboxil is a medication that targets the replication process of influenza viruses. It inhibits the endonuclease activity of the polymerase acidic protein (PA) in viral ribonucleoprotein complexes. During viral replication, the influenza virus undergoes a "cap-snatching" process to obtain functional replicated RNA fragments for transcription. This process involves the removal of the 5' cap of host mRNA, which is catalyzed by the endonuclease activity of PA. Baloxavir marboxil works by binding to the endonuclease site of PA and blocking its activity. This prevents the "cap-snatching" process and subsequently inhibits viral replication. Without functional RNA fragments, the virus cannot synthesize new virions or produce stable mRNA. By targeting the replication process, baloxavir marboxil helps to reduce the spread and severity of influenza infections caused by strains IVA, IVB, and IVC. It disrupts the production of new viral particles and hinders the release of virions from infected cells by neuraminidases. 1
Pharmacokinetics
Baloxavir marboxil is a prodrug that undergoes rapid hydrolysis to form its active metabolite, baloxavir acid (BXA). Hydrolysis occurs not only in the small intestine but also in the blood and liver through the action of arylacetamide deacetylase. BXA is primarily metabolized by uridine monophosphate glucuronosyl transferase 1A3 (UGT1A3), resulting in the formation of the glucuronidated form of BXA. A small amount of BXA is oxidized by cytochrome 3A4 (CYP) to its sulfoxide form. Baloxavir marboxil weakly inhibits CYP 3A4, as well as CYP 2B6 and 2C8. Following oral administration, it exhibits linear pharmacokinetics with a long elimination half-life of 80-100 hours. The drug has high protein binding (93-95%) and a large distribution volume (494-625 L). Enterohepatic circulation contributes to the excretion of the glucuronide metabolite of BXA into the gastrointestinal tract, where it is further metabolized by glucuronidases. Approximately 80% of the administered dose is eliminated through feces, while 15% is excreted in urine. It should be noted that both BXM and BXA are weak inhibitors of P-glycoprotein transporters. 2
Side effects
Regarding side effects, baloxavir marboxil was well tolerated Only the following observations were made in previous study, and their frequency did not increase with dose escalation: headache (most frequent) and perturbations of blood composition (increase in alanine and aspartate aminotransferase, lactate dehydrogenase, white blood cells, and eosinophil counts). All these events were reversible after trial completion. It was concluded that the tolerance profile was excellent, with no severe adverse events or treatment-related deaths reported. Clinical data over side effects, however, showed rather questionable results: only volunteers receiving the lowest doses (6 mg) reported these effects, while at higher doses (up to 80 mg), no such observations were made. The authors provided no tentative explanations for this. Furthermore, the subsequent CAPSTONE-1 study (phases II and III) provided similar results. To be complete, it should be mentioned that in the package insert and other references based on communications from Shionogi, the most significant side effects were as follows: diarrhea (3%), bronchitis (2%), nausea (1%), nasopharyngitis (1%), and headache (1%), all of them in the same range as for the placebo. Interestingly, the incidence of these effects was lower than in patients treated with OMV. 3
Reference
1. Dufrasne F. Baloxavir Marboxil: An Original New Drug against Influenza. Pharmaceuticals (Basel), 2021, 15(1):28.
2. Koshimichi H, Tsuda Y, Ishibashi T, Wajima T. Population Pharmacokinetic and Exposure-Response Analyses of Baloxavir Marboxil in Adults and Adolescents Including Patients With Influenza, J Pharm Sci, 2019, 108(5):1896-1904.
3. Hayden FG, Sugaya N, Hirotsu N, Lee N, de Jong MD, Hurt AC, Ishida T, Sekino H, Yamada K, Portsmouth S, Kawaguchi K, Shishido T, Arai M, Tsuchiya K, Uehara T, Watanabe A; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N Engl J Med, 2018, 379(10):913-923.
Related articles And Qustion
Lastest Price from Baloxavir marboxil manufacturers

US $0.00/kg2025-06-20
- CAS:
- 1985606-14-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $10.00/ASSAYS2025-05-04
- CAS:
- 1985606-14-1
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton