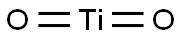Application and Synthesis of Titanium dioxide
General description
Titanium dioxide is an odorless white powder. Tasteless. pH 7.5. Occurs in three crystalline forms. (NTP, 1992). Chemicals Titanium dioxide is a titanium oxide with the formula TiO2. A naturally occurring oxide sourced from ilmenite, rutile and anatase, it has a wide range of applications. It has a role as a food colouring. Titanium dioxide, also known as titanium(IV) oxide or titania, is the naturally occurring oxide of titanium. It is used as a pigment under the names titanium white, Pigment White 6 (PW6), or CI 77891. It is typically extracted from ilmenite, rutile and anatase. Titanium dioxide is generally divided into anatase (type A) and rutile (type R).
Application
Titanium dioxide is an inorganic substance with the chemical formula of TiO2. It is a white solid or powdered amphoteric oxide with a molecular weight of 79.9. It is non-toxic and has the best opacity, whiteness and brightness. It is considered to be a white pigment with the best performance in the world today. Titanium dioxide has strong adhesion, is not easy to change chemically, and is always snow-white. It is widely used in coating, plastic, papermaking, printing ink, chemical fiber, rubber, cosmetics and other industries. It has a high melting point and is also used to make refractory glass, glaze, enamel, clay, high temperature resistant experimental utensils, etc. At the same time, titanium dioxide has a good UV masking effect. It is often used as a sunscreen in textile fibers. Ultrafine titanium dioxide powder is also added into sunscreen cream to make sunscreen cosmetics. Titanium dioxide can be extracted from rutile by acid decomposition or decomposed from titanium tetrachloride. Titanium dioxide has stable properties and is widely used as a white pigment in paint. It has good covering ability, which is similar to lead white, but it will turn black unlike lead white; It is as persistent as zinc white. Titanium dioxide is also used as a matting agent for enamel, which can produce a very bright, hard and acid resistant enamel surface.
1.Titania’s high refractive index and reactivity towards UV light are remarkable properties not only in the sunscreen industry, but also in food processing. The oxide’s nanoform has been developed for use in packaging light-sensitive food products, to ensure food durability. In this regard, merging TiO2 nanoparticles and biopolymers has given rise to “Active Food Packaging (AFP)”. AFP displays increased mechanical strength and antimicrobial properties compared to other "green" wrapping materials, such as paper, which is more sensitive to tearing, attracts moisture, and allows microbes to proliferate. The biolayer is usually chitosan, or a derivative that has an intrinsic biocidal activity. The biolayer is permeable to water and oxygen, which are adsorbed on the package’s surface, diffusing through it. In the presence of UV light, TiO2 transforms the diffused molecules into reactive oxygen species. These species target, oxidise, and inactivate microbes that would otherwise proliferate in food products ROS were also found to contribute towards the photodegradation of ethylene, a highly active growth regulator for fruits and vegetables. As such, fresh food discolouration and accelerated food softening are also minimized[1].
2.Another recent design in this field is represented by water-suspended magnesium micromotors coated with Au-doped titanium dioxide. When irradiated with UV light, the dispersed catalyst generates ROS from water molecules and inactivates Bacillus globigii, a simulant for the biological warfare agent Bacillus anthracis. The same micromotors were found to decompose organophosphate nerve agents into nonhazardous fragments, indicating an advantageous dual use[2].
Synthesis
1.Gas phase oxidation method: dry oxygen is used for gas phase oxidation at 923k-1023k:
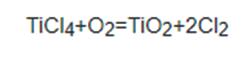
Figure 1 the Gas phase oxidation method
2.Sulfuric acid method: firstly, ground ilmenite and sulfuric acid (concentration ≥ 80%, temperature 343k-353k) are used to react under the condition of continuous air introduction and stirring to prepare soluble sulfate:
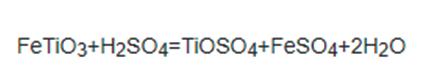
Figure 2 the Step1 of Sulfuric acid method
Because this reaction is exothermic, the temperature can reach 473k when the reaction is intense. When preparing titanium dioxide, the key step is to hydrolyze the titanium solution: the concentration and acidity temperature of the titanium solution.
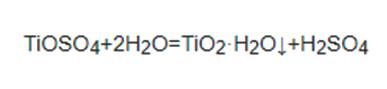
Figure the Step2 of Sulfuric acid method
Step2 will affect the hydrolysis reaction. The smaller the concentration, the smaller the acidity and the higher the temperature, the easier the reaction will occur. In order to raise the temperature, the reaction is often carried out under pressure, which can also make the precipitated particles more compact, so that the product has better physical properties.
Safety
In spite of the extensively use of TiO2-NPs, the biological effects and the cellular response mechanisms are still not completely elucidated and thus a deep understanding of the toxicological profile of this compound is required. The main mechanism underlining the toxicity potentially triggered by TiO2-NPs seems to involve the reactive oxygen species (ROS) production, resulting in oxidative stress, inflammation, genotoxicity, metabolic change and potentially carcinogenesis. The extent and type of cell damage strongly depend on chemical and physical characteristics of TiO2-NPs, including size, crystal structure and photo-activation. In this mini-review, we would like to discuss the latest findings on the adverse effects and on potential human health risks induced by TiO2-NPs exposure[2].
Recent breakthroughs are summarised herein, focusing on whether restructuring the surface properties of titanium dioxide either enhances or inhibits its reactivity , depending on the required application. Its recent exposure as a potential carcinogen to humans has been linked to controversies around titanium dioxide’s toxicity; this is discussed by illustrating discrepancies between experimental protocols of toxicity assays and their results. In all, it is important to review the latest achievements in fast-growing industries where titanium dioxide prevails, while keeping in mind insights into its disputed toxicity. At present, it is also difficult to assess what titania toxicity entails. Its dual behaviour towards light can mask the real experimental outcomes of toxicity assays that are based on the optical detection of cell viability parameters. This fact is concerning because many toxicity assays require protocolar adjustments for light-sensitive catalysts. In this respect, modern meta-analytical tools provide useful insights, by screening databases of experimental reports. Such analyses could guide future scientists on what experimental conditions need special calibrations for titanium dioxide and how to achieve them in the laboratory[1].
Reference
1.Racovita A. D., "Titanium Dioxide: Structure, Impact, and Toxicity," International Journal of Environmental Research and Public Health, Vol.19, No.9(2022), p.5681.
2.Grande F. & Tucci P., "Titanium Dioxide Nanoparticles: a Risk for Human Health?" Mini reviews in medicinal chemistry, Vol.16, No.9(2016), p.762.
You may like
Related articles And Qustion
See also
Lastest Price from Rutile manufacturers

US $1800.00-1600.00/ton2025-04-21
- CAS:
- 1317-80-2
- Min. Order:
- 0.001ton
- Purity:
- 99%
- Supply Ability:
- 5000

US $3.60/kg2025-04-18
- CAS:
- 1317-80-2
- Min. Order:
- 1kg
- Purity:
- ≥98%
- Supply Ability:
- 3000tons/month