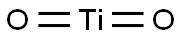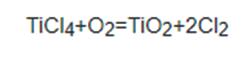Rutile: Polymorphism, Optical Properties and Nanoarchitecture Achievements
General Description
Rutile, a polymorph of titanium dioxide, stands out for its structural stability, high density, and refractive index. It is favored in various industries for its superior properties, especially in pigments and optical components. Rutile's high refractive index enables efficient light scattering, making it ideal for applications like paints and coatings. Its photoelectric behavior allows for catalyzing redox reactions under UV light, showcasing its potential in environmental purification and energy conversion technologies.

Figure 1. Rutile
Polymorphism
Titanium dioxide exists in three phases: as rutile, anatase, and brookite. These crystal phases assemble as octahedra, where six oxygen anions are shared by three titanium (IV) cations, hence the formula TiO6/3, which equals TiO2. While expanding in a three-dimensional space, these octahedral units arrange and distort differently for each polymorph, which leads to distinct patterns of crystallinity. As such, the three polymorphs differ in shape, structure, density, and refractive index. Rutile has a comparatively higher structural stability, given that transitions of this phase during synthesis and use are rare. The metastable anatase and brookite can be thermally restructured into the more thermodynamically stable rutile, depending on the mineral's industrial purpose. Brookite is a rarely encountered crystal phase and challenging to manufacture in industry.1
Optical Properties
Titania is a white powder with a high refractive index that averages values of 2.7 in rutile and 2.5 in anatase. The refractive index is a measure of the metal oxide’s ability to scatter photons. In this sense, rutile reflects light more efficiently compared to anatase and brookite, which marks rutile as an optimal pigment. Interestingly, the metal oxide has a dual behaviour towards light, by scattering and absorbing ultraviolet radiation, both highly desired properties in photocatalysis.
Under ultraviolet (UV) light, the metal oxide is a powerful semiconductor, capable of catalysing redox reactions due to its photoelectric properties. These relate to titania’s band structure. The energy gap (EG) is the region between the valence band, where the lower energy levels are occupied by valence electrons, and the conduction band, where higher energy levels can be filled by more electrons. Within a band gap, there are no energy levels for accommodating electrons. The value of EG is either equal to or smaller than the energy of ultraviolet photons. The region can be associated with a barrier for electron transfer—for higher EG values, stronger UV light must be absorbed by electrons in the valence band to ensure their excitation to energy levels within the conduction band. Specifically, titanium dioxide displays EG values of 3.1 eV for rutile and 3.3 eV for anatase. By illuminating TiO2 with ultraviolet light, electrons transit from their ground state in the valence band to an excited state in the conduction band (e−CB), giving rise to positive holes in the valence band (h+VB). After this event, molecules adsorbed onto the catalyst’s surface, such as water and oxygen, can undergo redox reactions. Oxidation occurs at the valence band, through h+VB electron acceptors, whereas reduction takes place at the conduction band, with the aid of elevated photoelectrons, e−CB.1
Nanoarchitecture Achievements
Rutile nanoparticles are part of the top five nanoparticles used in industry, owing to their versatility in applications—as photocatalysts, in pharmaceuticals, processed foods and household products, cosmetic white pigments, fabrics, and paints, and sunscreens.
Compared to microparticles, rutile nanoparticles display enhanced catalytic activity. This is because a decrease in size leads to an increased surface area available for catalysis. Recent breakthroughs have been achieved in medicinal applications of photocatalysis, by testing nano-titania as an anticancer agent. Balachandran et al. reported that irradiated TiO2 particles below 20 nm are an efficient "photo-killer" of pulmonary cancer cells. Valence band holes, with their strong oxidant character, lead to the formation of reactive oxygen species; these will interact with defective cells, causing significant intracellular damage, to finally induce their necrosis.1
References:
[1] RACOVITA A D. Titanium Dioxide: Structure, Impact, and Toxicity[J]. International Journal of Environmental Research and Public Health, 2022. DOI:10.3390/ijerph19095681.You may like
Related articles And Qustion
See also
Lastest Price from Rutile manufacturers

US $1800.00-1600.00/ton2025-04-21
- CAS:
- 1317-80-2
- Min. Order:
- 0.001ton
- Purity:
- 99%
- Supply Ability:
- 5000

US $3.60/kg2025-04-18
- CAS:
- 1317-80-2
- Min. Order:
- 1kg
- Purity:
- ≥98%
- Supply Ability:
- 3000tons/month