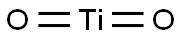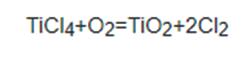What is Rutile?
Basic Informations
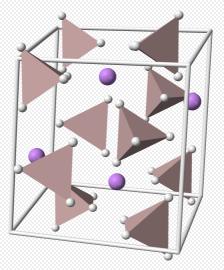
Rutile is one of the major minerals containing titanium. It is tetragonal and often has intact tetra-columnar or needle crystalline-like structure. Its aggregate exhibits granular or compacting blocky shape. It appears as dark, red, maroon, yellow or orange color with iron-rich product appearing as black color; it streaks appears as yellow to light brown color. It has adamantine gloss. Iron rutile exhibits semi-metallic gloss. It is brittle with the hardness being 6 to 6.5 and the density being 4.2~4.3 g/cm3. Products being rich in iron, niobium and tantalum have the density being increased with the high value being up to 5.5 g/cm3 or more. It can be dissolved in hot phosphoric acid. After cooling and dilution, adding sodium peroxide can turn the solution to brown color (titanium reaction). Rutile can be produced in gneiss, pegmatite, eclogite (flash) rock and placer.

Occurrence
Rutile is a common accessory mineral in high-temperature and high-pressure metamorphic rocks and in igneous rocks.
Thermodynamically, rutile is the most stable polymorph of TiO2 at all temperatures, exhibiting lower total free energy than metastable phases of anatase or brookite.[5] Consequently, the transformation of the metastable TiO2 polymorphs to rutile is irreversible. As it has the lowest molecular volume of the three main polymorphs, it is generally the primary titanium-bearing phase in most high-pressure metamorphic rocks, chiefly eclogites.
Within the igneous environment, rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those such as kimberlites and lamproites that have deep mantle sources. Anatase and brookite are found in the igneous environment particularly as products of autogenic alteration during the cooling of plutonic rocks; anatase is also found in placer deposits sourced from primary rutile.
The occurrence of large specimen crystals is most common in pegmatites, skarns, and granite greisens. Rutile is found as an accessory mineral in some altered igneous rocks, and in certain gneisses and schists. In groups of acicular crystals it is frequently seen penetrating quartz as in the fléches d'amour from Graubünden, Switzerland. In 2005 the Republic of Sierra Leone in West Africa had a production capacity of 23% of the world's annual rutile supply, which rose to approximately 30% in 2008.
Rutile can be used for the production of titanium dioxide, titanium sponge, titanium alloys, synthetic rutile, titanium tetrachloride, titanyl sulfate, potassium hexafluorotitanate and aluminum chloride or titanium chloride. Titanium dioxide can be used for making high-grade white paint, white rubber, synthetic fibers, paint, welding electrodes and the light reducing agent of rayon as well as the filler of plastics and advanced paper. It can also be applied to telecommunications equipment, metallurgy, printing, dyeing, enamel and other departments. Rutile is also the main mineral raw materials for extraction of titanium. Titanium and its alloys have many excellent properties including high strength, low density, excellent anti-corrosion properties, high temperature resistance, low temperature resistance and non-toxicity; it also has special features such as absorbing gases and superconductivity, and therefore is widely used in various kinds of fields including aviation, chemicals, light industry, navigation, medical, defense and marine resources development and so on. According to reports, more than 90% of the titanium mineral in the world has been used the production of titanium dioxide white pigment, and this product has more and more wide application in the paint, rubber, plastics, paper and some other industries.
Rutile can be used for welding, refining of titanium and manufacturing of titanium dioxide.
Rutile can be used as reagents analysis as well as being used for the preparation of highly pure titanium salts and being applied to pharmaceutical industry.
Rutile can be used as the carrier of catalyst, photo-catalytic media and the protection media against UV radiation. It also has wide application in various kinds of filed such as the coatings, plastics, self-cleaning automotive glass, automotive mirrors, act wall glass, screen glass bulb, air purification materials, medicine, cosmetics, water treatment, tanning and ink and so on.
Production
Rutile is mostly from strip mining. The selection of ore for the primary deposit of Titanium can be divided into three stages including pre-selection (usual through magnetic separation and gravity separation), iron selection (using magnetic separation) and Titanium selection (gravity separation, magnetic separation, electrostatic separation and flotation); The ore selection of Ti-Zr sand mineral (mainly include beach placer, followed by inland placer) mineral processing can be divided into two steps including roughing selection and featured. In 1995, the mining department in Zhengzhou Institute of comprehensive utilization had applied magnetic separation-gravity selection-acid leaching process for ore selection on the large-scale rutile ore in Xixia of Henan Province and had already passed the trial production with various kinds of indicators reaching the domestic leading level.
Synthetic rutile
Synthetic rutile was first produced in 1948 and is sold under a variety of names. It can be produced from the titanium ore ilmenite through the Becher process. Very pure synthetic rutile is transparent and almost colorless, being slightly yellow, in large pieces. Synthetic rutile can be made in a variety of colors by doping. The high refractive index gives an adamantine luster and strong refraction that leads to a diamond-like appearance. The near-colorless diamond substitute is sold as "Titania", which is the old-fashioned chemical name for this oxide. However, rutile is seldom used in jewellery because it is not very hard (scratch-resistant), measuring only about 6 on the Mohs hardness scale.
As the result of growing research interest in the photocatalytic activity of titanium dioxide, in both anatase and rutile phases (as well as biphasic mixtures of the two phases), rutile TiO2 in powder and thin film form is frequently fabricated in laboratory conditions through solution based routes using inorganic precursors (typically TiCl4) or organometallic precursors (typically alkoxides such as titanium isopropoxide, also known as TTIP). Depending on synthesis conditions, the first phase to crystallize may be the metastable anatase phase, which can then be converted to the equilibrium rutile phase through thermal treatment. The physical properties of rutile are often modified using dopants to impart improved photocatalytic activity through improved photo-generated charge carrier separation, altered electronic band structures and improved surface reactivity.
You may like
Related articles And Qustion
See also
Lastest Price from Rutile manufacturers

US $0.00-0.00/KG2026-01-04
- CAS:
- 1317-80-2
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $1800.00-1600.00/ton2025-04-21
- CAS:
- 1317-80-2
- Min. Order:
- 0.001ton
- Purity:
- 99%
- Supply Ability:
- 5000