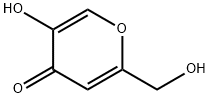
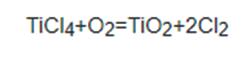The application of Kojic acid
General description
Kojic acid is a pyranone that is 4H-pyran substituted by a hydroxy group at position 5, a hydroxymethyl group at position 2 and an oxo group at position 4. It has been isolated from the fungus Aspergillus oryzae. It has a role as a NF-kappaB inhibitor, an Aspergillus metabolite, a skin lightening agent, an EC 1.10.3.1 (catechol oxidase) inhibitor, an EC 1.10.3.2 (laccase) inhibitor, an EC 1.13.11.24 (quercetin 2,3-dioxygenase) inhibitor, an EC 1.14.18.1 (tyrosinase) inhibitor and an EC 1.4.3.3 (D-amino-acid oxidase) inhibitor. It is an enol, a primary alcohol and a member of 4-pyranones. It derives from a hydride of a 4H-pyran. Kojic acid is a natural product found in Marrubium cylleneum, Aspergillus terricola, and Aspergillus candidus with data available.
Application and Pharmacology
Melasma is a common skin condition that affects many patients with hyperpigmented patches on sun-exposed facial skin. It disproportionately affects people of Asian, African, and Hispanic descents. Unfortunately, there are not many lasting and effective treatment options available, which has led many practitioners and skincare companies to trial various topical ingredients. In the past several years, kojic acid has gained popularity as a skincare ingredient to treat hyperpigmentation and melasma. Kojic acid has more recently become popular in over-the-counter skincare products. We discuss the background of kojic acid, its suggested mechanism, and the available studies evaluating its utility in treating melasma.
Skin color disorders can be caused by various factors, such as excessive exposure to sunlight, aging and hormonal imbalance during pregnancy, or taking some medications. Kojic acid (KA) is a natural metabolite produced by fungi that has the ability to inhibit tyrosinase activity in synthesis of melanin. The major applications of KA and its derivatives in medicine are based on their biocompatibility, antimicrobial and antiviral, antitumor, anti-diabetic, anticancer, anti-speck, anti-parasitic, and pesticidal and insecticidal properties. In addition, KA and its derivatives are used as anti-oxidant, anti-proliferative, anti-inflammatory, radio protective and skin-lightening agent in skin creams, lotions, soaps, and dental care products. KA has the ability to act as a UV protector, suppressor of hyperpigmentation in human and restrainer of melanin formation, due to it tyrosinase inhibitory activity. Also, KA could be developed as a chemo sensitizer to enhance efficacy of commercial antifungal drugs or fungicides. In general, KA and its derivatives have wide applications in cosmetics and pharmaceutical industries[1,2].
Synthesis and its derivatives
Two novel kojic acid derivatives, kojicones A and B (1 and 2), along with the precursors kojic acid (3) and (2R,4R)-4-hydroxy-5-methoxy-2,4-dimethyl-2- [(2R)-2-methylbutyryloxy]-5-cyclohexen-1,3-dione (4), were isolated from a fungal strain Aspergillus versicolor. Their structures and absolute configurations were accurately confirmed by HRESIMS data, NMR analysis, and electronic circular dichroism (ECD) calculations. Kojicones A and B were the first examples of kojic acid adducts with cyclohexen-1,3-dione possessing unprecedented tricycle skeletons. Compounds 1–3 were found to have inhibition on the NO production of murine RAW 264.7 cells. They can also reduce the mRNA expression of four cytokines (IL-6, IL-1β, TNF-α, and iNOS) and promote the expression of IL-4 at 20 μM. Moreover, kojic acid (3) could treat the DSS (dextran sulfate sodium)-induced colitis on mice with the effectiveness similar to that of the positive control. The results suggested that kojic acid and its derivatives could be a promising anti-inflammatory source for the medicinal and cosmetic industry[3].
Safety
Kojic acid is an organic compound with molecular formula of c6h6o4 and molecular weight of 142.11. Colorless prismatic crystal. Used as an antioxidant and anti radiation agent. On October 27,2017, the list of carcinogens published by the international agency for research on cancer of the World Health Organization was preliminarily sorted out for reference. Kojic acid was included in the list of three types of carcinogens.
Reference
1.Saeedi M., Eslamifar M. & Khezri K., "Kojic acid applications in cosmetic and pharmaceutical preparations," Biomedicine & Pharmacotherapy, Vol.110(2019), pp.582-593.
2.Lachowicz J. I., Todde D. & Aberamchuk K. et al., "Kojic acid derivatives as double face ligands for metal and phosphate ions.," Journal of Inorganic Biochemistry, Vol.222(2021), p.111520.
3.Li T., Liang J. & Liu L. et al., "Novel kojic acid derivatives with anti-inflammatory effects from Aspergillus versicolor," Fitoterapia, Vol.154(2021), p.105027.
You may like
See also
Lastest Price from Kojic acid manufacturers
US $0.00-0.00/kg2025-08-21
- CAS:
- 501-30-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00-0.00/KG2025-05-26
- CAS:
- 501-30-4
- Min. Order:
- 1KG
- Purity:
- ≥99.0%
- Supply Ability:
- 5tons/month