8-Hydroxyquinoline - Reaction / Application on synthetic works
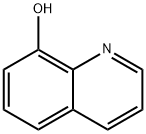
8-Hydroxyquinoline is an organic compound with the formula C9H7NO. It is a derivative of the heterocycle quinoline by placement of an OH group on carbon number 8. This light yellow compound is widely used to synthetize substituted quinoline products.
The following example is about its application on the synthesis of Azo-8-hydroxyquinoline dyes [1]
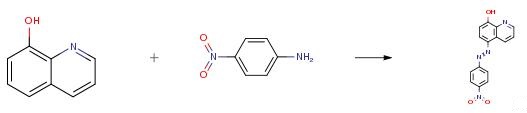
2 mmol carbocyclic amine was dissolved in HCl (1.5 mL) and water (4 mL). The solution was cooled in an ice-salt bath and a cold solution of NaNO2 (0.15 g, 2 mmol) in water (3.0 mL) was added dropwise with stirring. The resulting diazonium salt was also cooled in an ice-salt bath. Excess nitrous acid was destroyed by the addition of urea. And then added dropwise with stirring to 8-hydroxyquinoline (0.29 g, 2 mmol) in KOH (2 mmol, 0.112 g) and water (2 mL), cooled in an ice-salt bath. The solution was stirred at 0-5 °C for 1 h and the pH of the reaction mixture was maintained at 4-6 by the simultaneous addition of saturated sodium acetate solution (15-20 mL). The mixtures were stirred for further 1 h. The resulting solid was filtered off, washed with cold water and dried. The obtained compounds were purified by crystallization using ethanol.
The following example is about its application on the synthesis of azo dye complexes [2]
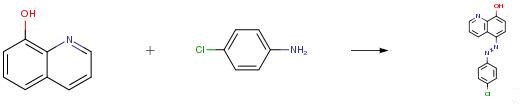
25 ml of distilled water containing 0.01 mol hydrochloric acid are added to aniline (0.01 mol) or p-derivatives. A solution of 0.01 mol sodium nitrite in 20 ml of water is added dropwise to the resulting mixture then stirred and cooled to 0 °C. The formed diazonium chlorides consecutively coupled with an alkaline solution of 0.01mol quinoline-8-ol, in 10 ml of pyridine. The colored precipitate, which formed immediately, is filtered through sintered glass crucible and washed several times with water. The crude products are purified by recrystallization from hot ethanol and dried in vacuum desiccator over P2O5. The products are also characterized by IR, 1H and 13C NMR spectroscopy and elemental analysis. Yield percent was 65%.
The following example is about its application on the synthesis of aryl tosylates and mesylates [3]
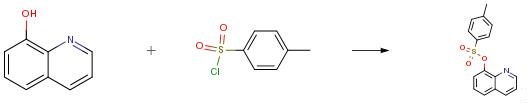
To a solution of hydroxyarene (25.0 mmol) in THF (15 mL) was added 10 percent K2CO3 (65.00 g, 47.1 mmol) or 15 percent NaOH (22.00 g, 82.5 mmol). After the resulting solution was cooled to 0 °C with an ice-water bath, a solution of TsCl (4.82 g, 25.3 mmol for 10 percent K2CO3 or 5.72 g, 30.0 mmol for 15 percent NaOH) in THF (35 mL) was slowly added within 15 min at 0 °C. After addition of TsCl, the ice-water bath was removed and the reaction mixture was stirred for 2 h. To the mixture was then added ethyl acetate (100 mL; more was needed when aryl tosylate has poor solubility in ethyl acetate). The two-phase mixture was separated. The organic layer was washed with water (50 mL) and dried over anhydrous MgSO4. Removal of solvent under reduced pressure gave the corresponding pure aryl tosylate. Any trace amount of TsCl, if present, can be removed by simply washing the product with hexanes.
References
1.Saylam A, Seferoǧlu Z, Ertan N. Azo-8-hydroxyquinoline dyes: The synthesis, characterizations and determination of tautomeric properties of some new phenyl- and heteroarylazo-8-hydroxyquinolines[J]. Journal of Molecular Liquids, 2014, 195:267 – 276.
2.El-Sonbati D, El-Bindary, Shoair B. Thermal properties, geometrical structures, antimicrobial activity and DNA binding of supramolecular azo dye complexes[J]. Journal of Molecular Liquids, 2016, 218:400-420.
3.Lei X, Jalla A, Abou S, Mhd AS, Jamie M, Cao B. Chromatography-Free and Eco-Friendly Synthesis of Aryl Tosylates and Mesylates[J]. Synthesis, 2015, 47(17): 2578 – 2585.
Related articles And Qustion
Lastest Price from 8-Hydroxyquinoline manufacturers

US $0.00/KG2025-04-21
- CAS:
- 148-24-3
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1.00/g2025-04-21
- CAS:
- 148-24-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 100kg
![1674334-59-8 3-Bromo-11,11-dimethyl-11H-benzo[b]fluorene; applications; reaction; synthesis](/NewsImg/2024-09-03/6386098117280318013736304.png)

![1674334-59-8 3-Bromo-11,11-dimethyl-11H-benzo[b]fluorene; applications; reaction; synthesis](httpss://www.chemicalbook.com/NewsImg/2019-11-27/201911279562810645.jpg)
