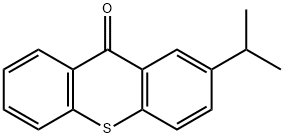
2-Isopropylthioxanthone - Reaction / Application on synthetic works
2-Isopropylthioxanthone is a compound, which can play the role of a sulphur type photoinitiator in printing industries and UV-cured inks for food packaging materials.[1] It is also an important organic intermediate to synthetize substituted thioxanthone products.
The following example is about its application on the synthesis of new multifunctional cationic photoinitiators[1]

10.0 g (0.03937 moles) of 2-isopropylthioxanthone were dissolved in 630 ml of a mixture of acetonitrile and water (75 percent acetonitrile, 25 percent water by volume). Gentle heating was required to dissolve the 2-isopropylthioxanthone (35°C). The temperature was then allowed to return to room temperature. 86.336 g of Ceric ammonium nitrate (0.15748 moles) were added in one batch. The reaction was followed by TLC (thin layer chromatography). The reaction mixture was stirred for 2.5 hours at room temperature. 400 ml of water was then added and the mixture was extracted with 1000 M1 of diethyl ether. The ether layers were combined and dried with magnesium sulphate, and the ether was removed on a rotary evaporator to yield the product. At this stage the product still contained some inorganic residue. The product was therefore re-dissolved in diethyl ether, washed with water and dried with magnesium sulphate. The ether was then removed on a rotary evaporator to yield the product. Product yield 5.54 g (52.3percent) of a yellow solid.
The following example is about its application on the synthesis of model thioxanthone compounds[2]

Under nitrogen protection, to a solution containing elemental bromine (15.98 g, 100.00 mmol) in dichloromethane (50 ml) at room temperature was added ZnCl2 (0.68 g,4.99 mmol). The mixture was cooled to 0 ° C. And then 2 hours slowly added by adding 2-isopropyl thioxanthone (12.70g,49.93 mmol) of 50 ml of methylene chloride solution. After completion of the reaction, the reaction was stirred under cooling with cold water for 24 hours, with dichloromethane extraction (3×100 mL), saturated brine and water, The organic layer was dried over anhydrous sodium sulfate and the solvent was evaporated under reduced pressure at 60 ° C, Add 100 ml of methanol to the oar, and filter to give 8.15 g of a white solid powder product.
The following example is about its application on the synthesis of a modified new photoinitiator. [3]

To a 5000 ml, 3-necked, round-bottomed flask equipped with overhead mechanical stirrer, reflux condenser connected to a sodium hydroxide scrubber and nitrogen inlet was dissolved a solution of 2-ITX (700 g, 2.752M) in thionyl chloride (3262 g, 2000 ml, 27.42M, 10 Mol eq's). Under a nitrogen stream this wine-red solution was heated to reflux (internal temp: 80° C.) with stirring for 4.5 hours. The reaction mixture was cooled to ambient overnight under a positive pressure of nitrogen. The wine red reaction mixture was transferred to a rotorvapor and excess thionyl chloride was removed under reduced pressure (water bath temperature was maintained at 40° C.). The mobile oil was taken up in toluene (1050 ml) and then added drop wise, under a nitrogen atmosphere, over 1.5 hour, to a previously prepared and vigorously stirred solution of sodium methoxide (595 g, 11.014M, 4.0 Mol eq's) in methanol (2100 ml) at such a rate as to keep the vessel temperature between 0-10° C. Once addition was complete, the cooling bath was removed and the vessel contents allowed to warm to ambient over 1 hour. Water was added to the cooling bath and stirring continued for a further hour. The reaction mixture was then transferred to a rotorvapor and methanol removed under reduced pressure. The resulting residue was quenched into a vigorously stirred mixture of toluene (1.0 l) and water (3.5 L). Stirring was continued for 20 minutes at ambient and the reaction mixture was then transferred to a separating funnel and allowed to settle over 15 minutes. The toluene layer was back washed with water (1.75 L) and allowed to separate. The upper toluene layer was removed from the separator, dried over sodium sulphate and stored in the freezer overnight. The Toluene solution was then filtered through Celite and the resulting orange/brown filtrate was transferred to a rotorvapor and toluene removed under reduced pressure to give crude 9,9-Dimethoxy-2isopropylthioxanthone as a pale brown oil.
References
1.Sun Chemical Corporation. Herlihy SL, Rowatt B, Davidson RS. Multifunctional cationic photoinitiators, their preparation and use. WO2004/55000[P], 2004, A1, Page 18-19.
2.Tongji University. Jin M, Wu X, Pan H, Wan D. Model thioxanthone compounds and use thereof (by machine translation). CN106995430[P], 2017, A, Paragraph 0046-0049.
3.Lintfield Limited. Johnstone RAW, Loureiro RM da Silva. Photoinitiated reactions. US2013/105297[P], 2013, A1, Paragraph 0156; 0157.
You may like
Related articles And Qustion
Lastest Price from 2-Isopropylthioxanthone manufacturers

US $0.00/kg2025-10-28
- CAS:
- 5495-84-1
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $0.00/KG2025-04-21
- CAS:
- 5495-84-1
- Min. Order:
- 20KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month