3-Bromo-11,11-dimethyl-11H-benzo[b]fluorene - Reaction / Application on synthetic works
3-Bromo-11,11-dimethyl-11H-benzo[b]fluorene is an important organic intermediate to synthetize substituted benzo[b]fluorene products.
The following example is about its application on the synthesis of organic electroluminescent device [1]
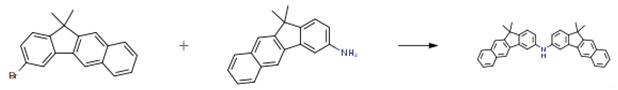
3-Amino-11,11-dimethyl-11H-benzoindole (2.59 g, 10 mmol) was added to the reactor.3-bromo-11,11-dimethyl-11H-benzoxanthene (3.23 g, 10 mmol),Pd2(dba)3 (0.21 g, 0.25 mmol), P(t-Bu)3 (0.18 g, 0.84 mmol), NaOt-Bu (2.8 g, 25 mmol),100 mL of toluene solution, reacted at 100 ° C for 24 h, After the reaction, the organic phase was extracted with diethyl ether and water. The organic layer was dried over MgSO4, and concentrated organics, by column chromatography, Recrystallization gave the product (3.96 g, 79 percent).
The following example is about its application on the synthesis of an electroluminescent compound [2]

4 g of 3-Bromo-11,11-dimethyl-11H-benzo[b]fluorene (12 mmol), 5.1 g of starting material (12 mmol), 0.57 g of tris(dibenzylideneaceton)dipalladium(0) (0.6 mmol), 0.51 g of SPhos (1.2 mmol), 3.0 g of sodium tert-butoxide (3.1 mmol), and 60 mL of toluene were introduced into a reaction vessel, and the mixture was refluxed for 1 hour. The reaction solution was cooled to room temperature, and the solvent was then removed by a rotary evaporator. The residue was purified by column chromatography to obtain 6.1 g of the product (yield: 75percent).
The following example is about its application on the synthesis of an organic light-emitting device [3]

Under an argon atmosphere, to the amine (4.36 g, 10 mmol), 3-bromo-11,11-dimethyl-11H-benzo[b]fluorene (3.35 g, 10 mmol), tris(dibenzylideneacetone)dipalladium (0.14 g, 0.15 mmol), tri-tert-butylphosphine tetrafluoroborate (0.087 g, 0.3 mmol), sodium tert-butoxide (1.9 g, 20 mmol) was added with 50 ml of anhydrous xylene and refluxed for 8 hours. The mixture was cooled to 50 ° C, filtered through Celite / silica gel, and the filtrate was concentrated. The crude product obtained was recrystallized from toluene. The product (2.95 g, 6.6 mmol) was obtained in 66% yield.
The following example is about its application on the synthesis of an organic electroluminescent device [4]
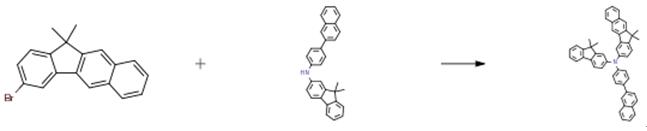
7.0 g of 3-bromo-11,11-dimethyl-11H-benzo[b]fluorene (22 mmol), 9.8 g of the amine (24 mmol), 0.6 g of tris(dibenzylideneaceton)dipalladium(0) (0.66 mmol), 0.6 mL of tri-tert -butylphosphine (1.32 mmol, 50 percent toluene solution), 3.1 g of sodium tert-butoxide (32mmol), and 110 mL of toluene were introduced into a reaction vessel, and the mixture was refluxed for 1 hour. The reaction solution was cooled to room temperature, and the solvent was then removed by a rotary evaporator. The residue was purified by column chromatography to obtain 2.8 g of the product (yield: 20 percent).
References
1.Changchun Hai Purunsi Technology Co., Ltd. Dong X, Cai H. An aromatic amine derivative and its organic electroluminescent device (by machine translation). CN109336834[P], 2019, A, Paragraph 0078-0080.
2.Rohm And Haas Electronic Materials Korea Ltd. Oh HS, Lee T, Kim YK, M DH, Lim YM, Hong JR. Organic electroluminescent compound and organic electroluminescent device comprising the same. WO2019/4587[P], 2019, A1, Paragraph 187; 188; 189.
3.Changchun Hai Purunsi Technology Co., Ltd. Han C, Cai H. A three-aryl amine compound and its organic light-emitting device (by machine translation). CN109293516[P], 2019, A, Paragraph 0095; 0096; 0098
4.Rohm And Haas Electronic Materials Korea Ltd. Oh HS, Lee TJ, Kim YK, Moon DH, Lim YM, Hong JR. Organic electroluminescent compound and organic electroluminescent device comprising the same. WO2019/4587[P], 2019, A1, Paragraph 157; 158; 159.
You may like
Related articles And Qustion
See also
Lastest Price from 3-Bromo-11,11-dimethyl-11H-benzo[b]fluorene manufacturers
![1674334-59-8 3-Bromo-11,11-dimethyl-11H-benzo[b]fluorene](/ProductImageEN/2022-09/Small/dde249ef-1227-4f15-a34d-1f4787543a2a.png)
US $0.00-0.00/KG2025-04-04
- CAS:
- 1674334-59-8
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton
![1674334-59-8 3-Bromo-11,11-dimethyl-11H-benzo[b]fluorine](/ProductImageEN/2023-12/Small/72aceff5-9b35-47bc-8ebd-a8327740143f.png)
US $185.00-1333.00/g2025-02-08
- CAS:
- 1674334-59-8
- Min. Order:
- 5g
- Purity:
- 0.99
- Supply Ability:
- 10kg
![1674334-59-8 3-Bromo-11,11-dimethyl-11H-benzo[b]fluorene; applications; reaction; synthesis](https://www.chemicalbook.com/CAS/20180529/GIF/1674334-59-8.gif)