What is the mechanism of action of 8-Hydroxyquinoline
Introduction
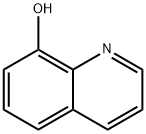
8-Hydroxyquinoline (8HQ), a quinoline derivative originating in plants and from synthesis, has been used as a fungicide in agriculture and a preservative in the textile, wood, and paper industries. 8HQ possesses potent coordinating ability and good metal recognition properties, which means it is widely used for analytical and separation purposes and metal chelation[1].

Mechanism of action
8-Hydroxyquinoline is a partial inhibitor of RNase and is occasionally added to organic extraction buffers that contain phenol. As an antioxidant, 8-hydroxyquinoline stabilizes phenol and retards the formation of quinones (phenol oxidation products). It is usually added to a final concentration of 0.1% (w/v). 8-Hydroxyquinoline imparts a bright yellow colour to the phenol-chloroform to which it is added, thereby helping the investigator keep track of the organic and aqueous phases during the nucleic acid purification process[2].
8-Hydroxyquinoline also chelates heavy metals, making it very useful for removing VDR from cell lysates. Upon binding VDR, the 8-hydroxyquinoline changes from yellow to dark green, and repeated extractions with 8-hydroxyquinoline-containing phenol: chloroform are often necessary. When the phenol-containing phase of the extraction buffer remains yellow, all VDRs have been removed. Including 8-hydroxyquinoline in organic extracting buffers may also be advantageous even when VDR is not used because heavy metals can cause RNA degradation when they are present with RNA for extended periods. Needless to say, all reagents should have been prepared using high-purity, nuclease-free water.
Biological activity
Specifically, 8-hydroxyquinoline has been shown to have antimicrobial activity against various pathogens, including fungi and Gram-positive and -negative bacteria. For example, 8HQ has been found to kill Mycobacterium tuberculosis (MTB) in a copper-dependent manner, both in vitro with copper added and by itself in macrophages, which provides copper. This finding is significant as it tips the scales to benefit macrophages, specifically alveolar macrophages, which are charged to clear this respiratory pathogen as MTB tries to survive within them by inhibiting phagosome–lysosome fusion. Of further significance, The Shah et al. paper showed that 8HQ was able to kill both replicating and nonreplicating forms of the notably slow-growing pathogen.
Unfortunately, 8HQ is toxic to mammalian hosts in moderate concentrations, thus complicating its use. To circumvent this issue, one study added a protective pinanediol boronic ester group to 8HQ, for which its cleavage was sensitive to hydrogen peroxide. Upon entering the activated macrophage phagolysosome, the reactive oxygen species present removed the boronic group, leading to significantly improved tolerability in the animal model versus 8HQ and enhanced Cryptococcus neoformans killing. This study provides proof of concept for modifying metal ionophores to have activity at the clearance site, thus reducing off-target effects and toxicity of compounds. Of note, the 8HQ analogue 5,7-dichloro-2-[(dimethylamino)methyl]quinolin-8-ol (PBT2), which has prior approval for use against Alzheimer’s disease, is a zinc ionophore that has been used to sensitize resistant Gram-negative and -positive bacteria to killing by traditional antibiotics.
[1] Veda Prachayasittikul. “8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications.” Drug Design, Development and Therapy 7 (2013): 1157–78.
[2] 8-Hydroxyquinoline - an overview | ScienceDirect Topics https://www.sciencedirect.com/topics/immunology-and-microbiology/8-hydroxyquinoline.
References:
[1] VEDA PRACHAYASITTIKUL. 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications.[J]. Drug Design, Development and Therapy, 2013, 7. DOI:10.2147/DDDT.S49763.You may like
Related articles And Qustion
Lastest Price from 8-Hydroxyquinoline manufacturers

US $0.00/KG2025-04-21
- CAS:
- 148-24-3
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1.00/g2025-04-21
- CAS:
- 148-24-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 100kg