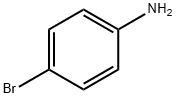
4-Bromoaniline: synthesis and applications in organic synthesis
General Description
The synthesis of 4-Bromoaniline involves N-TBS protection to shield the aniline nitrogen, using mild conditions and eco-friendly 2-MeTHF solvent. This protected form is useful in Heck cross-coupling reactions, catalyzed by Pd nanocrystals on COFs, to form C-C bonds efficiently. Additionally, 4-Bromoaniline acts as an aromatic amine in Mannich reactions, enabling the introduction of bromine into β-amino ketones for further chemical modifications. These processes are significant in producing pharmaceuticals and complex organic molecules, with 4-Bromoaniline being a versatile intermediate for various synthetic applications.
Synthesis
The synthesis of 4-Bromoaniline involves a highly efficient and chemoselective process for the protection of aniline derivatives. The key step in this synthesis is the N-TBS (tert-butylsilyl) protection of the aniline nitrogen atom. This process is achieved under exceptionally mild conditions, utilizing methyllithium as a deprotonating agent to activate the aniline nitrogen for subsequent reaction with the TBS-Cl (tert-butylsilyl chloride). The reaction takes place in 2-methyltetrahydrofuran (2-MeTHF), which serves as an eco-friendly alternative to the traditional solvent tetrahydrofuran (THF). 2-MeTHF is preferred due to its lower toxicity and environmental impact. The procedure is carried out at room temperature and requires only 30 minutes for completion, highlighting its efficiency. Following the N-TBS protection, the 4-bromoaniline derivative can be further manipulated as needed for various chemical syntheses. If the protecting group needs to be removed, an efficient method is available where the N-TBS-anilines are treated with silica gel in a mixture of ethanol and water, allowing for the cleavage of the TBS group and regeneration of the free aniline. In summary, the synthesis of 4-Bromoaniline via N-TBS protection is a straightforward, mild, and environmentally conscious approach that provides an effective route to protected aniline derivatives, with the option of easy deprotection when necessary. 1
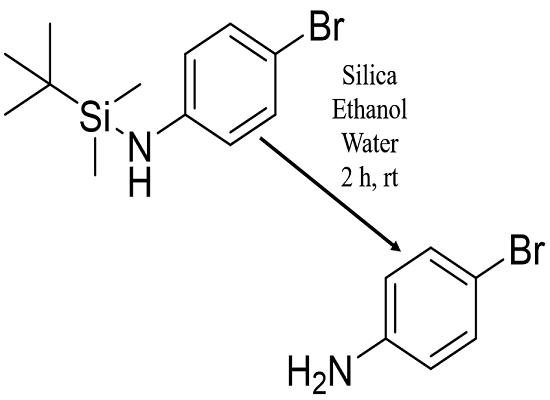
Figure 1. Synthesis of 4-bromoaniline
Applications in organic synthesis
Aryl halide substrate
4-Bromoaniline, a brominated derivative of aniline, is a compound that can be used in the synthesis of various organic molecules through catalytic processes. 4-Bromoaniline could serve as an aryl halide substrate in Heck cross-coupling reactions, which are facilitated by palladium nanocrystals (Pd NCs) supported on covalent organic frameworks (COFs). These COFs are synthesized using tricarboxylic acids and triazine moieties and provide a structured environment for the in-situ growth of Pd NCs with a controlled particle size of 1-5 nm. The Pd NCs@COFs catalyst demonstrates high efficiency in promoting carbon-carbon (C-C) bond formation, achieving complete conversion (100%) when used with vinyl derivatives and aryl halides such as 4-Bromoaniline. The catalytic system's effectiveness is further evidenced by its good stability and ability to maintain high catalytic activity over multiple cycles. Such catalytic applications of 4-Bromoaniline are crucial in the production of pharmaceuticals, agrochemicals, and other fine chemicals, showcasing the importance of integrating nanotechnology and organic synthesis for industrial advancements. 2
Mannich reaction
4-Bromoaniline can be utilized as the aromatic amine component in the three-component Mannich reaction. This reaction involves the condensation of an aromatic aldehyde, an alkyl (hetero)aryl ketone, and an aromatic amine like 4-Bromoaniline, catalyzed by camphor-10-sulfonic acid under solvent-free conditions. The significance of 4-Bromoaniline in this process lies in its ability to introduce a bromine substituent into the resulting β-amino ketone product. The presence of the bromine atom on the aromatic ring can further serve as an advantageous site for subsequent chemical transformations, due to its reactivity towards various nucleophilic substitution reactions. For example, the bromine atom can be displaced by other nucleophiles, or it can participate in cross-coupling reactions to create more complex molecules. In the specific application to the synthesis of aminochromans, the Mannich adduct derived from 4-Bromoaniline could potentially undergo an intramolecular etherification, leading to the formation of aminochroman structures. These aminochroman compounds are of interest due to their potential biological activities and their presence in various natural products and pharmaceuticals. Thus, 4-Bromoaniline serves as a key building block in the construction of such valuable heterocyclic compounds. 3
Reference
1. Pace V, Alcantara AR, Holzer W. Highly efficient chemoselective N-TBS protection of anilines under exceptional mild conditions in the eco-friendly solvent 2-methyltetrahydrofuran. Green Chemistry, 2011, 13(8): 1986-1989.
2. Palladium nanocrystals-embedded covalent organic framework as an efficient catalyst for Heck cross-coupling reaction. Microporous and Mesoporous Materials, 2022, 339, 111961.
3. (±)-Camphor-10-sulfonic acid catalyzed direct one-pot three-component Mannich type reaction of alkyl (hetero)aryl ketones under solvent-free conditions: application to the synthesis of aminochromans. RSC Advances, 2012, 2(2): 480-486.
Related articles And Qustion
See also
Lastest Price from 4-Bromoaniline manufacturers
US $1.00/kg2025-03-14
- CAS:
- 106-40-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $20.00-8.00/kg2025-03-07
- CAS:
- 106-40-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons