4-Bromoaniline: properties, applications and safety
General Description
4-Bromoaniline is a versatile organic compound with distinct physical and chemical properties. It finds utility in the production of azo dyes, dihydroquinazolines, para-bromobiphenyl, and as a supplement in bacterial culture media. However, it poses potential risks to human health, causing harm if swallowed, skin and eye irritation, respiratory irritation, and organ damage through prolonged or repeated exposure. Hence, appropriate safety measures, such as wearing protective clothing and ensuring adequate ventilation, should be followed when handling the compound. Overall, 4-Bromoaniline's unique combination of reactivity and solubility makes it a valuable building block in the development of new chemical compounds, while proper safety protocols must be observed to minimize its potential harm.

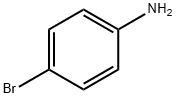
Figure 1. 4-Bromoaniline
Properties
4-Bromoaniline is an organic compound with the molecular formula C6H6BrN. It is a derivative of aniline, where one of the hydrogen atoms on the benzene ring is replaced by a bromine atom. This substitution results in significant changes in the chemical and physical properties of the compound. One of the notable properties of 4-Bromoaniline is its solid state at room temperature, appearing as colorless to pale yellow crystals. It has a melting point of around 69-71°C, which allows for easy handling and purification. The compound is soluble in organic solvents such as ethanol and acetone but has limited solubility in water. In terms of reactivity, 4-Bromoaniline exhibits typical characteristics of an aromatic amine. It can undergo various chemical reactions including electrophilic aromatic substitution, oxidation, and coupling reactions. The bromine atom attached to the benzene ring makes it more susceptible to nucleophilic attack compared to aniline. Overall, 4-Bromoaniline is a versatile compound with distinct properties that make it useful in organic synthesis and other applications such as pharmaceuticals and dyes. Its unique combination of reactivity and solubility makes it a valuable building block for the development of new chemical compounds. 1
Applications
4-Bromoaniline is a versatile compound with various applications. Primarily, it serves as a crucial precursor in the synthesis of azo dyes and dihydroquinazolines. Azo dyes are widely used in industries such as textiles, printing, and coloring agents due to their vibrant colors and excellent colorfastness. The presence of the bromine atom in 4-Bromoaniline enhances the stability and color properties of the resulting dyes. Furthermore, 4-Bromoaniline finds utility as a carbon and nitrogen supplement in the culture medium of Moraxella sp. strain G, a type of bacterium. This compound provides essential nutrients for the growth and metabolism of the bacterium, enabling researchers to study its characteristics and potential applications. Additionally, 4-Bromoaniline plays a vital role in the synthesis of para-bromobiphenyl through the Gomberg-Bachmann reaction. Para-bromobiphenyl is a valuable intermediate compound used in the production of liquid crystals, pharmaceuticals, and agrochemicals. Moreover, 4-Bromoaniline acts as a building block in the preparation of pharmaceutical and organic compounds. Its bromine substituent allows for further functionalization, enabling the introduction of specific chemical groups or modifications to create desired properties or activities in the target compounds. Overall, 4-Bromoaniline's applications span from the production of dyes and intermediates to its use as a supplement in bacterial culture media, making it a valuable compound in the fields of chemistry, materials science, and biotechnology. 2
Safety
4-Bromoaniline is a hazardous compound that poses potential risks to human health. It can cause harm if swallowed, skin and eye irritation, respiratory irritation, and organ damage through prolonged or repeated exposure. Therefore, appropriate safety precautions, such as wearing protective clothing and using respiratory protective equipment, should be followed when handling the compound. Adequate ventilation should also be ensured in areas where it is used or stored. By adhering to proper safety protocols, the potential harm from 4-Bromoaniline can be minimized, ensuring safe handling and use of the compound. 3
Reference
1. PubChem. COMPOUND SUMMARY: 4-Bromoaniline. National Library of Medicine, 2005, PubChem CID: 7807.
2. Siddiqa A, Zubair M, Bilal M, Rasool N, Qamar MU, Khalid A, Ahmad G, Imran M, Mahmood S, Ashraf GA. Synthesis of Functionalized N-(4-Bromophenyl)furan-2-carboxamides via Suzuki-Miyaura Cross-Coupling: Anti-Bacterial Activities against Clinically Isolated Drug Resistant A. baumannii, K. pneumoniae, E. cloacae and MRSA and Its Validation via a Computational Approach. Pharmaceuticals (Basel). 2022 Jul 8;15(7):841.
3. Summary of Classification and Labelling: 4-bromoaniline.European Chemicals Agency, EC / List no. 203-393-9.
You may like
Related articles And Qustion
See also
Lastest Price from 4-Bromoaniline manufacturers
US $1.00/kg2025-03-14
- CAS:
- 106-40-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $20.00-8.00/kg2025-03-07
- CAS:
- 106-40-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons