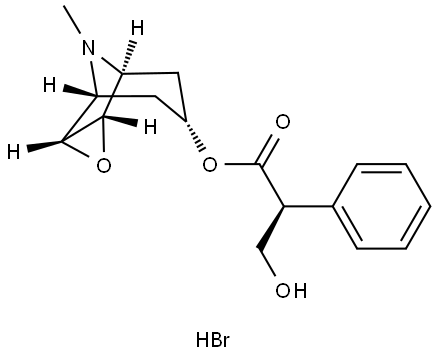
Scopolamine Hydrobromide: Beyond PONV/Motion Sickness
Scopolamine hydrobromide is a medication used to manage and treat postoperative nausea and vomiting (PONV) and motion sickness. It is in the anticholinergic class of drugs. This activity will highlight the indications, mechanism of action, adverse event profile, and other key factors (e.g., off-label uses, dosing, pharmacodynamics, pharmacokinetics, monitoring, relevant interactions) pertinent for members of the healthcare team in the care of patients with PONV and related conditions. The most commonly reported side effects of scopolamine hydrobromide patch use are blurred vision, dilated pupils, and dry mouth. The vision disturbances are most often due to inadequate handwashing techniques after the application of the patch. Less frequently reported side effects are related to anticholinergic toxidrome: flushed skin, tachycardia, agitation, and confusion. These side effects are usually mild and quick to resolve after patch removal. If needed, the clinician can administer a reversal agent like physostigmine if a side effect persists. Toxicity does not happen as frequently with the transdermal form of scopolamine hydrobromide due to its extended-release nature. Data on the toxic dose of scopolamine in the tablet form is scattered. Reports exist that 10 mg a day can be lethal for children.

Scopolamine hydrobromide Produces Larger Antidepressant and Antianxiety Effects
Major depressive disorder (MDD) is reported twice as often in women when compared with men. The basis for this gender difference remains unclear. Some propose that women are more willing to seek help and to report symptoms than are men, leading simply to a reporting bias. Others have suggested that gender-specific influences of socialization, acute stressors, or sexual assault may explain the higher prevalence of depression in women. Finally, biological theories suggest that differences in brain structure and/or function underlie the difference in prevalence. An insight into the underlying neurobiology of depression and mechanistic explanations for diagnostic subgroups will benefit from expanding our knowledge and understanding of gender differences. Recently, we reported that the antimuscarinic agent, Scopolamine hydrobromide, produced rapid and robust antidepressant effects in currently depressed male and female patients with MDD or bipolar disorder (BD). The purpose of this study was to determine if gender-based differences exist in the antidepressant response to scopolamine. The antidepressant and antianxiety responses to Scopolamine hydrobromide were evaluated by assessing changes in MADRS and HAM-A scores, respectively. The CGI assessed overall clinical improvement. Secondary outcome measures included the VAS and the POMS to assess acute changes in mood within each session.[1]
Scopolamine hydrobromide produced improvement in depression severity by the first assessment following the first administration of the drug in both gender groups; thus, the rapidity of the antidepressant response was comparable, but by study end the magnitude of response was greater in females than in males. In addition, a larger proportion of women showed a response to scopolamine (71%) than did men (38%), and thus the greater improvement was driven partially by the larger proportion of women who responded to scopolamine. The NMDAR gene expression is enhanced by muscarinic receptor stimulation in at least some brain structures, and thus the elevated muscarinic receptor sensitivity identified in mood disorders may contribute to an elevation in NMDAR transmission. Scopolamine hydrobromide administration reduces mRNA concentrations for NMDAR types 1A and 2A in rat brain and via this mechanism may reduce NMDAR function. Several features of the sample selection limit the generalizability of the current findings. First, the sample was relatively small for the male participants. Second, both elderly and pediatric subjects, and current nicotine users, were excluded from participation in the study, and thus the findings may not generalize to such cases. Finally, we used a single regimen for the administration of Scopolamine hydrobromide.
Scopolamine hydrobromide-induced “cholinergic stress test” in the elderly
A healthy, young-looking, intelligent and cheerful 80-year-old woman was evaluated at the Houston Methodist Hospital Memory Clinic because of an episode of “scopolamine intoxication” that occurred on November 12, 2010. She flew from Texas to Hawaii and prior to boarding a cruise ship applied a post-auricular transdermal Scopolamine hydrobromide patch to prevent seasickness. Her last memories of the vacation include arriving to Hawaii and boarding the cruise ship. She has no recollection after becoming confused, delirious, agitated, and experiencing visual hallucinations. Because of her markedly altered mental status she was disembarked, admitted to a local hospital in Hawaii and eventually flown back to Texas 5 days later.On November 30, 2010 her neurological and neuropsychological evaluation was consistent with Mild Cognitive Impairment - Amnestic type. A 2-deoxy-2-(18F)fluoro-D-glucose (FDG) positron emission tomography (PET) brain scan showed markedly increased tracer uptake in the basal ganglia bilaterally. We believe a cautionary note is required before granting unrestricted endorsement to this treatment. In our experience, the use of Scopolamine hydrobromide in the elderly may trigger episodes of amnestic delirium. The following case exemplifies the effect of transdermal scopolamine for prevention of motion sickness during a vacation ship cruise by a previously normal elderly woman.[2]
The transdermal scopolamine patch applied to the post-auricular area contains 1.5 mg of scopolamine; the system releases in-vivo approximately 1.0 mg of scopolamine over a 72-h period with an average plasma concentration of 87 pg/mL of free scopolamine; detectable levels are found within 4 h with a peak level at 24 h. Scopolamine is a high-affinity selective competitive antagonist of G protein-coupled muscarinic receptor for acetylcholine. Scopolamine hydrobromide penetrates the blood-brain barrier, readily blocking cholinergic transmission in the CNS. It not only affects the vestibular nuclei and the vomiting center, but also the extensive cholinergic network that originates in the Nucleus Basalis of Meynert. We hypothesize that the CNS cholinergic blocking effect of scopolamine induces a “cholinergic stress test” resulting in amnestic delirium in elderly patients who have low central cholinergic reserves as a result of age, subclinical Alzheimer's disease, or vascular lesions affecting the cholinergic pathways in the periventricular white matter. It is well demonstrated that the elderly are more sensitive to the cognitive effects of scopolamine than young individuals, probably due to central cholinergic system deficits associated with aging. Age is also relevant in the reversible reduction of cerebral blood flow and glucose consumption produced by cholinergic blockage induced by Scopolamine hydrobromide.
An unintentional overdose of scopolamine
Scopolamine hydrobromide (hyoscine) is an antimuscarinic drug which is primarily used in the prophylaxis and treatment of motion sickness and as a premedication to dry bronchial and salivary secretions. In acute overdosage, the main clinical problem is central nervous system (CNS) depression. In Australia, tablets containing scopolamine hydrobromide 0.3 mg are available over the counter in packs of ten. The recommended dose for adults is one to two tablets as a single dose, repeated four to six hours later, if required. The maximum dose stated on the pack is four tablets over a 24-hour period with a caution regarding drowsiness and blurred vision. We describe a patient who presented with symptoms of anticholinergic syndrome secondary to an unintentional overdose of scopolamine. Whilst at work, the patient noticed that he had forgotten his prescribed medication, domperidone, at home; a friend gave him some travel sickness medication which contained Scopolamine hydrobromide for relief of nausea. On a previous occasion, he had experienced a similar, less severe reaction with another anticholinergic agent, loperamide. This report highlights the need to consider nonprescription products, ie, over the counter medications, herbal/nutritional supplements as causes of anticholinergic syndrome when a patient presents with symptoms suggestive of this diagnosis.[3]
References
[1]Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010 Nov;35(12):2479-88. doi: 10.1038/npp.2010.131. Epub 2010 Aug 25. PMID: 20736989; PMCID: PMC3055321.
[2]Román GC, Jackson RE, Longoria EM, Fisher RE. Scopolamine-induced "cholinergic stress test" in the elderly. Front Pharmacol. 2014 Aug 13;5:182. doi: 10.3389/fphar.2014.00182. PMID: 25165458; PMCID: PMC4131233.
[3]Corallo CE, Whitfield A, Wu A. Anticholinergic syndrome following an unintentional overdose of scopolamine. Ther Clin Risk Manag. 2009 Oct;5(5):719-23. doi: 10.2147/tcrm.s6732. Epub 2009 Sep 15. PMID: 19774213; PMCID: PMC2747390.
See also
Lastest Price from Scopolamine hydrobromide manufacturers
US $0.00-0.00/G2025-09-29
- CAS:
- 114-49-8
- Min. Order:
- 10G
- Purity:
- 99
- Supply Ability:
- 100KG

US $5.00-0.50/KG2025-05-08
- CAS:
- 114-49-8
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS