Some methylglyoxal related diseases
Introduction
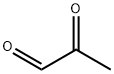
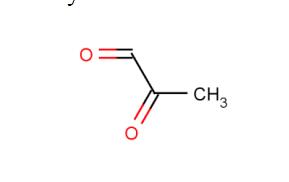
Methylglyoxal (MGO;Figure 1) is the major compound belonging to α-dicarbonyl molecules,which are termed “reactive carbonyl species” (RCS) responsible for “carbonyl stress”. They are highly reactive compounds that easily modify biological macromolecules, including peptides, proteins, lipoproteins, and nucleic acids via the generation of advanced glycation end products (AGEs). Therefore, together with other irritable molecules like reactive oxygen and nitrogen species (RONS), they disturb the functioning of cellular organelle,thus stimulating their rearrangements, leading to autophagy, apoptosis, or proliferation of cells. Such phenomena, when not counteracted by detoxifying mechanisms, stimulate oxidative stress and enhance inflammatory processes, contributing to the development of a variety of pathological conditions. Methylglyoxal upregulation, followed by deleterious effects at the cellular and systemic levels, is associated with metabolic disturbances (hyperglycemia/hyperinsulinemia/insulin resistance/hyperlipidemia/inflammatory processes/carbonyl stress/oxidative stress/hypoxia). Therefore, it is implicated in a variety of disorders, including metabolic syndrome, diabetes mellitus, and cardiovascular diseases.[1]

As novel upstream regulator of DNA methylation
Aerobic glycolysis, also known as the Warburg effect, is predominantly upregulated in a variety of solid tumors, including breast cancer. Methylglyoxal (MG), a very reactive by-product of glycolysis, unexpectedly enhanced the metastatic potential in triple negative breast cancer (TNBC) cells. Methylglyoxal and Methylglyoxal-derived glycation products have been associated with various diseases, such as diabetes, neurodegenerative disorders, and cancer. Glyoxalase 1 (GLO1) exerts an anti-glycation defense by detoxifying MG to D-lactate. In this study, Dube et al. used our validated model consisting of stable GLO1 depletion to induce methylglyoxal stress in TNBC cells. Using genome-scale DNA methylation analysis, we report that this condition resulted in DNA hypermethylation in TNBC cells and xenografts. GLO1-depleted breast cancer cells showed elevated expression of DNMT3B methyltransferase and significant loss of metastasis-related tumor suppressor genes, as assessed using integrated analysis of methylome and transcriptome data. Interestingly, MG scavengers revealed to be as potent as typical DNA demethylating agents at triggering the re-expression of representative silenced genes. Importantly, we delineated an epigenomic methylglyoxal signature that effectively stratified TNBC patients based on survival. Conclusion: This study emphasizes the importance of methylglyoxal oncometabolite, occurring downstream of the Warburg effect, as a novel epigenetic regulator and proposes methylglyoxal scavengers to reverse altered patterns of gene expression in TNBC.[2]
Methylglyoxal stress arises in diabetes and the mechanisms driving CKD
Chronic kidney disease (CKD) remains a serious diabetic complication despite the use of widely employed interventions such as angiotensin-converting enzyme inhibitors and glucose-lowering treatments.Accumulation of methylglyoxal, a highly reactive glucose metabolite and a major precursor in the formation of advanced glycation end products, may link the hemodynamic, inflammatory, metabolic, and structural changes that drive diabetic CKD. Therefore, methylglyoxal may serve as a potential therapeutic target to prevent diabetic CKD.Recent findings Higher plasma methylglyoxal levels were shown to be associated with a decline in the estimated glomerular filtration rate. Furthermore, interventions that lower methylglyoxal levels reduced albuminuria in rodent models of diabetes. In addition, the glyoxalase system, which detoxifies methylglyoxal into D-lactate,has been identified as a key protective enzymatic system against diabetic CKD in both human and rodent studies. Recently, several promising treatments to lower methylglyoxal directly or to boost the glyoxalase system have been identified.
In contrast to cardiovascular disease, the incidence of ESRF has not substantially declined in people with diabetes over the last two decades. Identification of novel modifiable risk factors for CKD is therefore important. A large body of experimental research has identified methylglyoxal and GLO1 as key players in the development of CKD in diabetes. Methylglyoxal has been linked to adverse hemodynamic,inflammatory, metabolic, and structural changes in the kidney. The glyoxalase pathway seems to be a gatekeeper for the prevention of high methylglyoxal levels leading to kidney damage. Recent work has confirmed a link between higher methylglyoxal levels and vascular and renal disease in human studies. Moreover, several compounds targeting the glycation pathway are under active investigation. Particularly, the GLO1 activator and the methylglyoxal quenching compounds pyridoxamine and alagebrium as well as sevelamer carbonate are of interest, as they seem to be well tolerated in humans. Furthermore, dietary interventions reducing the intake of carbonyls and AGEs may prove valuable in preventing CKD. Taken together, these efforts on glycation research may reduce the burden of CKD in people with diabetes.[3]
Methylglyoxal in Cardiometabolic Disorders
Pathological features characteristic of metabolic syndrome and diabetes (insulin resistance, hyperglycemia, hypertension, dyslipidemia, and obesity) increase the risk of cardiovascular disease (CVD).The mechanisms that link increased glycolytic flux with endothelial and, hence, cardiovascular impairment consider the stimulation of side pathways of glycolysis leading to the overproduction of sorbitol (polyol pathway), generation of glucosamine-6-phosphate (hexosamine pathway), and overproduction of trioses due to the inhibition of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Triose accumulation leads to the activation of protein kinase C (PKC) (by overproduced DAG), as well as MAGEs generation (due to the excessive generation of methylglyoxa).Triose-derived dicarbonyl molecules (mainly Methylglyoxal) show much greater efficiency in AGE formation in comparison to glucose. Therefore, they modify intra- and extracellular proteins, the latter being components both of ECM and plasma proteins. These actions impair the functionality of blood vessels, e.g., via decreasing their elasticity through the disturbances in collagen's structure or stimulating prooxidative,proinflammatory, and procoagulatory processes (induced by blood plasma AGEs binding with their receptors on macrophages or endothelial cells). The upregulation of AGEs receptors (RAGEs) and their ligands being observed under hyperglycemic conditions further adds to ROS and inflammation enhancement, contributing to vascular endothelium destruction.[1]
References
1. Berdowska I, Matusiewicz M, Fecka I. Methylglyoxal in Cardiometabolic Disorders: Routes Leading to Pathology Counterbalanced by Treatment Strategies. Molecules. 2023;28(23):7742. Published 2023 Nov 24. doi:10.3390/molecules28237742
2.Dube G, Tiamiou A, Bizet M, et al. Methylglyoxal: a novel upstream regulator of DNA methylation. J Exp Clin Cancer Res. 2023;42(1):78. Published 2023 Mar 31. doi:10.1186/s13046-023-02637-w
3.Hanssen NMJ, Stehouwer CDA, Schalkwijk CG. Methylglyoxal stress, the glyoxalase system, and diabetic chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28(1):26-33. doi:10.1097/MNH.0000000000000465
You may like
Related articles And Qustion
See also
Lastest Price from Methylglyoxal manufacturers
US $100.00/KG2025-04-21
- CAS:
- 78-98-8
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON

US $30.00-10.00/KG2025-04-15
- CAS:
- 78-98-8
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg