Isosorbide:introduction,synthesis and application
Introduction
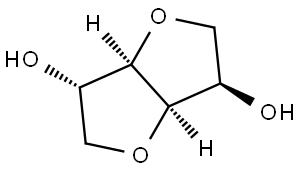
Isosorbide, also known as d-isosorbide, 1,4:3,6-dianhydro-d-sorbitol, and 1,4-dianhydrosorbitol, has white color and crystalline appearance, and high hydrophilicity.(Figure 1) It is from the family of diols with a unique two fused furan rings with two secondary hydroxy groups in puckered confirmation (angle of 120°). The C2 hydroxy group extends outside the ring system (exo), and the C5 hydroxy group sits inside the “V”shape of the rings (endo), with the latter participating in hydrogen bonding with the adjacent THF ring.This hydrogen bond is thought to be at least partially responsible for higher reactivity typically observed for the endo hydroxy group. The endo-position hydroxy group is sterically shielded and more acidic than the exo-position hydroxy group.The difference in acidity is explained by the intra-molecular hydrogen-bonding between the endo-hydroxy group and the closely located furanic oxygen. This difference between the two hydroxy groups of isosorbide allows selective mono-functionalization of the diol. Isosorbide is one of the most interesting cellulosic-derived molecules with great potential to be implemented in wide range of products that shaping our daily life. According to the recent United Nation sustainability plans and European Union vision for sustainable and carbon neutral societies by 2030, isosorbide from non-edible wet cellulosic biomass will be one of the central bio-based building blocks for the production of fine chemicals and biodegradable polymers.[1]

Isosorbide synthesis
Typically, isosorbide is synthetized from sorbitol via a double acid-catalyzed dehydration reaction.Sorbitol is a lignocellulosic biomass-derived platform chemical, which is obtained from the sugar fraction of biomass, mostly from starch and cellulose. To produce sorbitol, starch (or cellulose) are hydrolyzed to glucose, which is hydrogenated toward sorbitol in the following step, generally applying Raney nickel as acatalyst.Nonetheless, the transition toward sustainable biorefinery processes should also include the technological process required. In this respect, IUPAC concluded in 2019 that flow chemistry is one of the top ten emerging technologies that will contribute to fulfil the United Nation’s Sustainable Development Goals (SDGs), by the year 2030. Flow chemistry processes, with respect to batch counterparts, eventually minimize the risk of handling hazardous and dangerous substances with low cost and increase process efficiency with minimal waste generation, both preventing harm and lowering the environmental impact. Furthermore, flow chemistry allows novel chemical transformations to occur that are not possible in batch.[1]
Application study
Isosorbide as a Renewable Platform chemical[2]
Isosorbide is a platform chemical of considerable importance for the future replacement of fossil resource-based products.Applications as monomers and building blocks for new polymers and functional materials, new organic solvents, for medical and pharmaceutical applications, and even as fuels or fuel additives are conceivable. The conversion of isosorbide to valuable derivatives by functionalization or substitution of the hydroxyl groups is difficult because of the different configurations of the 2- and 5-positions and the resulting different reactivity and steric hindrance of the two hydroxyl groups.
Isosorbide is an ideal, versatile platform chemical and has attracted significant interest in recent years. Compounds derived by further conversion, for example, by etherification or esterification reactions, might be of interest for applications as high-boiling green solvents, fuels, or fuel additives, surfactants, and others,which depend on the type of substituent and its chain length. Isosorbide itself provides versatile possibilities for applications as a monomer in new polymers. The hydroxyl groups can be converted into other functional groups, which lead to a huge variety of monomers for the production of polymeric materials.Additionally, a large amount of multifunctional aliphatic alcohols and alkanes of different chain lengths (≤6) is accessible starting from sorbitol via isosorbide by dehydration/hydrogenation and provides another wide field of important platform chemicals.
Although a substantial amount of work has been published using exclusively the endo or exo derivatives isomannide and isoidide, respectively, as starting material, a considerable effortis still necessary to transfer and adapt these methods for the efficient conversion of isosorbide.
References
1. Brandi F, Al-Naji M. Sustainable Sorbitol Dehydration to Isosorbide using Solid Acid Catalysts: Transition from Batch Reactor to Continuous-Flow System. ChemSusChem. 2022;15(5):e202102525. doi:10.1002/cssc.202102525
2. Rose M, Palkovits R. Isosorbide as a renewable platform chemical for versatile applications--quo vadis?. ChemSusChem. 2012;5(1):167-176. doi:10.1002/cssc.201100580
You may like
See also
Lastest Price from Isosorbide manufacturers

US $8000.00/KG2026-01-31
- CAS:
- 652-67-5
- Min. Order:
- 1000KG
- Purity:
- ≥99.5%
- Supply Ability:
- 250MT/month

US $5.00-0.50/KG2025-05-08
- CAS:
- 652-67-5
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS