3-Chloroperoxybenzoic acid – Properties, Preparation and Application
Chemical Properties
3-Chloroperoxybenzoic acid is also known as 3-chloroperoxybenzoic acid, MCPBA and m-chloroperbenzoic acid. The molecular weight of 3-chloroperbenzoic acid is 172.57. 3-chloroperoxybenzoic acid is white powdery crystals. Melting point is 92-94°C (decomposed). It is almost insoluble in water, but soluble in ethanol, ethers, chloroform, and dichloroethane. It is thermally stable and has an annual decomposition rate of less than 1% at room temperature. The decomposition rate is accelerated in the liquid state. 3-Chloroperbenzoic acid is sensitive to heat and shock, and pure solid. 3-Chloroperbenzoic acid is flammable and potentially explosive. It contains a weak –O–O– bond and a nucleophilic OH group, that makes it versatile oxidative and easily breakable. The chlorobenzoic acid peroxide content in the commercial products is 70%, 75%, 80%, 85%, 95%, so it will contain a certain amount of water and m-chlorobenzoic acid, and it will appear as a slightly moist powder.
Preparation method
The preparation method is a mixed solution of magnesium sulfate heptahydrate, sodium hydroxide, hydrogen peroxide, and dioxane, stirring in an ice water bath, and adding m-chlorobenzoyl chloride. After the reaction, the product was neutralized by adding cold sulfuric acid to obtain 3-chloroperoxybenzoic acid.
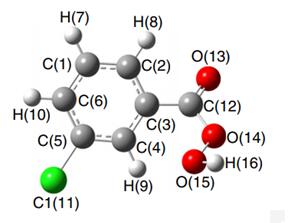
Fig 1. Molecular structure of 3-chloroperoxybenzoic acid [1].
Applications
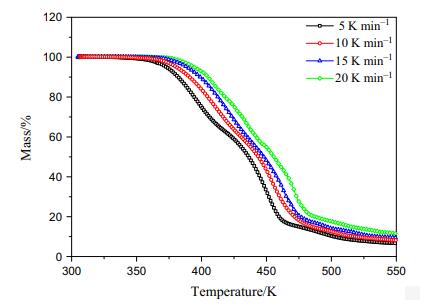
Fig 2. TG curves of 3-Chloroperoxybenzoic acid as an oxidant for olefin epoxidation
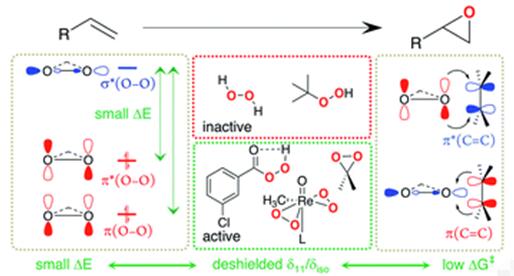
Fig 3. 3-Chloroperoxybenzoic acid as an oxidant for olefin epoxidation
3-Chloroperoxybenzoic acid is commonly used in double bond epoxidation, nitridation, cyclization, Baeyer-Villiger oxidation, and N-oxidation. It can also be used as an oxidant for fine chemicals such as synthetic medicine and pesticides. It is also sometimes used as a bleaching agent [1-6].
• Used in cyclization reaction, Baeyer-Villiger reaction, N-oxidation reaction and S-oxidation reaction.
• Used as an oxidant for fine chemical products such as synthetic medicine and pesticides.
• Used as oxidant and bleach.
• As a good electrophilic reagent, it can react with many functional groups and can oxidize olefins, enol silyl ethers, furans, sulfides, selenides and amino compounds.
References
[1] Yang J , Jiang J , Jiang J , et al. Thermal instability and kinetic analysis on m-chloroperbenzoic acid[J]. 2019.
[2] Ehinger C, Gordon C P, Copéret C. Oxygen transfer in electrophilic epoxidation probed by 17 O NMR: differentiating between oxidants and role of spectator metal oxo[J]. Chemical science, 2019, 10(6): 1786-1795.
[3] Kureshy R I , Ahmad I , Khan N U H , et al. Chiral Mn(III) salen complexes covalently bonded on modified MCM-41 and SBA-15 as efficient catalysts for enantioselective epoxidation of nonfunctionalized alkenes[J]. Journal of Catalysis, 2006, 238(1):134-141.
[4] Yaremenko Ivan A, Vil’ Vera A, Demchuk Dmitry V, et al. Rearrangements of organic peroxides and related processes [J]. Beilstein Journal of Organic Chemistry, 12:1647-1748.
[5] Michalak M , Kawalec M , Kurcok P . Reactive mono- and di-epoxy-functionalized poly (3-hydroxybutyrate)s. Synthesis and characterization[J]. Polymer Degradation and Stability, 2012, 97(10):p.1861-1870.
[6] Tank R. m-Chloroperoxybenzoic acid (MCPBA). Synlett. 2007, 04:0664–5.
You may like
Related articles And Qustion
See also
Lastest Price from 3-Chloroperoxybenzoic acid manufacturers

US $9.90/KG2025-04-21
- CAS:
- 937-14-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $30.00-10.00/kg2024-10-11
- CAS:
- 937-14-4
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 100000kg